Clostridium difficile infection and fecal bacteriotherapy: Difference between revisions
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction: History and Significance== | ||
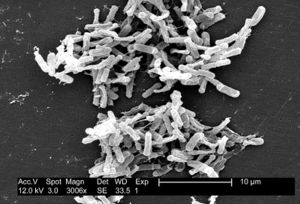
[[Image:Img_1.jpg|thumb|right|Scanning electron micrograph of C. difficile bacteria obtained from the CDC Public Health Image Library.]] | [[Image:Img_1.jpg|thumb|right|Scanning electron micrograph of C. difficile bacteria obtained from the CDC Public Health Image Library.]] | ||
<br>By Rebecca Varnell<br> | <br>By Rebecca Varnell<br> | ||
Revision as of 01:47, 22 April 2015
Introduction: History and Significance
By Rebecca Varnell
Clostridium difficile is a Gram-positive, spore-forming Firmicute that is found abundantly in nature (Figure 1). The rod-shaped bacteria was first noticed and described by Hall and O’Toole in 1935 because of its notable abundance in the intestine of infants (Poxton 2005). C. difficile can and often do live among the diverse microbiota of a healthy human and animal gut. They are found abundantly in soil as well as human and animal feces. However, in the absence of microbial competition, C. difficile can proliferate in individuals with compromised intestinal microbial communities such as in patients treated with heavy antibiotics. The bacterial lifecycle includes the formation of dormant spores, which can be passed from individuals infected with or carrying C. difficile by fecal-oral route. Dormant spores are known for their resilience and longevity, creating a public health crisis (Patel 2010). The presence and spread of C. difficile in hospitals and nursing homes is a major concern for doctors and patients alike.
In 1978, researchers first isolated C. difficile in the stools of individuals suffering from antibiotic-associated pseudomembranous colitis (PMC), a severe inflammation of the colon exhibited by individuals treated with heavy courses of antibiotics (Patel 2010). In the following decades, a series of studies investigated these bacteria. Since a well-known study in 1990 by Borriello et al. articulated the pathogenesis and urgency of C. difficile, this bacteria and its management has been studied extensively.
C. difficile infection (CDI) is the umbrella term that refers to colonization of C. difficile in a human host. CDI can present itself clinically in a diversity of ways, most notably antibiotic-associated diarrhea (AAD) and antibiotic-associated colitis (AAC) (Farooq et al. 2015). CDI is a major issue in the United States; it is one of the most common health-care associated infections in the US and most commonly affects hospitalized patients and individuals in nursing homes. Major CDI outbreaks in hospitals have caused healthcare providers to reevaluate liberal use of antibiotic treatment. Alternative treatment methods such as fecal microbiota therapy (FMT) that avoid the continued use of antibiotics may be the most sustainable, effective, and cost-efficient treatment for CDI (Boyle et al. 2015).
History and significance
Clostridium difficile is a Gram-positive, spore-forming Firmicute that is found abundantly in nature (Figure 1). The rod-shaped bacteria was first noticed and described by Hall and O’Toole in 1935 because of its notable abundance in the intestine of infants (Poxton 2005). C. difficile can and often do live among the diverse microbiota of a healthy human and animal gut. They are found abundantly in soil as well as human and animal feces. However, in the absence of microbial competition, C. difficile can proliferate in individuals with compromised intestinal microbial communities such as in patients treated with heavy antibiotics. The bacterial lifecycle includes the formation of dormant spores, which can be passed from individuals infected with or carrying C. difficile by fecal-oral route. Dormant spores are known for their resilience and longevity, creating a public health crisis (Patel 2010). The presence and spread of C. difficile in hospitals and nursing homes is a major concern for doctors and patients alike.
In 1978, researchers first isolated C. difficile in the stools of individuals suffering from antibiotic-associated pseudomembranous colitis (PMC), a severe inflammation of the colon exhibited by individuals treated with heavy courses of antibiotics (Patel 2010). In the following decades, a series of studies investigated these bacteria. Since a well-known study in 1990 by Borriello et al. articulated the pathogenesis and urgency of C. difficile, this bacteria and its management has been studied extensively.
C. difficile infection (CDI) is the umbrella term that refers to colonization of C. difficile in a human host. CDI can present itself clinically in a diversity of ways, most notably antibiotic-associated diarrhea (AAD) and antibiotic-associated colitis (AAC) (Farooq et al. 2015). CDI is a major issue in the United States; it is one of the most common health-care associated infections in the US and most commonly affects hospitalized patients and individuals in nursing homes. Major CDI outbreaks in hospitals have caused healthcare providers to reevaluate liberal use of antibiotic treatment. Alternative treatment methods such as fecal microbiota therapy (FMT) that avoid the continued use of antibiotics may be the most sustainable, effective, and cost-efficient treatment for CDI (Boyle et al. 2015).
Section 2
Include some current research, with at least one figure showing data.
Section 3
Include some current research, with at least one figure showing data.
References
[1] Nazarko, L. (2015). Infection control: Clostridium difficile. British Journal Of Healthcare Assistants, 9(1), 20-25.
Andres F Carrion et al. “Severe colitis associated with docetaxel use: a report of four cases,” World Journal of Gastrointestinal Oncology, 10 (2010): 390-394, accessed April 17, 2015, doi: 10.4251/wjgo.v2.i10.390.
Daniel E. Voth and Jimmy D. Ballard, “Clostridium difficile Toxins: Mechanism of Action and Role in Disease,” Clinical Microbiology Reviews 18 (2005): 249-263, date accessed April 19, 2015, doi: 10.1128/CMR.18.2.247-263.2005.
Daniel Paredes-Sabja et al. “Clostridium difficile spore biology: sporulation, germination, and spore structural proteins,” Trends in Microbiology 22 (2014): 406, accessed April 15, 2015, doi: 10.1016/j.tim.2014.04.003.