Conidiobolus coronatus: Difference between revisions
Alina.burns (talk | contribs) (Created page with "==Classification== ===Higher order taxa=== Domain: Eukaryota; Kingdom: Fungi; Phylum: Zygomycota; Subphylum: Zygomycotina Order: Entomophthorales; Family: Ancylistaceae; Genus...") |
No edit summary |
||
| (11 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Moore2013}} | |||
[[File: Conidia.gif|frame|200px|right|Spherical conidia with prominent papillae characteristic of ''Conidiobolus coronatus''. Image from [http://www.mycology.adelaide.edu.au/Fungal_Descriptions/Zygomycetes/Conidiobolus/ Mycology Online]]] | |||
==Classification== | ==Classification== | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Kingdom: Eukaryota | |||
Domain: Fungi | |||
Phylum: Entomophthoromycota | |||
Subphylum: Entomophthoramycotina | |||
Order: Entomophthorales | |||
Family: Ancylistaceae | |||
Genus: ''Conidiobolus'' | |||
===Species=== | ===Species=== | ||
Conidiobolus coronatus | |||
''Conidiobolus coronatus'' | |||
Synonym: ''Entomophthora coronata'', ''Delacroixia coronata'' | |||
{| | |||
| height="10" bgcolor="#FFDF95" | | |||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=34488 Taxonomy] | |||
|} | |||
==Description and significance== | ==Description and significance== | ||
C. coronatus was first described by Constantin in 1897 and first isolated by Emmons and Bridges | ''C. coronatus'' was first described by Constantin in 1897 in France and first isolated by Emmons and Bridges in 1961 in Australia <sup>1</sup>. It is commonly present in soil and decaying organic matter and has a world-wide distribution with a high prevalence in tropical and subtropical regions. | ||
''C. coronatus'' is the major causative agent of rare condition of rhinofacial entomophthoromycosis (conidiobolomycosis) in humans and other mammals, such as horses, dogs and llamas. It is also known to parasitize large variety of insects by rupturing the exoskeleton and killing the insect host within 1-2 days. | |||
==Genome structure== | ==Genome structure== | ||
{| | |||
| height="10" bgcolor="#FFDF95" align="center" | | |||
'''NCBI: [http://www.ncbi.nlm.nih.gov/genome/?term=conidiobolus%20coronatus Genome]''' | |||
|} | |||
[http://genome.jgi.doe.gov/cgi-bin/kogBrowser?db=Conco1 The DOE Joint Genome Institute] has sequenced draft assembly of ''C. coronatus NRRL28638'' strain genome using [http://en.wikipedia.org/wiki/454_Life_Sciences#Full_genome_sequencing_.28de_novo_sequencing_and_resequencing.29 454 technology]. | |||
The genome of contains 39,90 Mbp and has been partially annotated. Total gene count - 6929 genes. | |||
==Cell and colony structure== | ==Cell and colony structure== | ||
C. coronatus grows on potato dextrose, Sabouraud and cornmeal agar. The strains that are adapted to human infection readily grow at 37°C. Colonies of C. coronatus are fast growing, flat, waxy, creamy white to tan with age. As a colony becomes older it obtains powdery texture and the lid of the petri dish becomes covered with sticky conidia. The colonies grow quickly and can reach a diameter of 6 cm in 48 hours. The species are easy to maintain in the laboratory environment. | [[File: ColonyCC.jpg|300px|thumb|right|'Conidiobolus coronatus''colony on potato dextrose agar. 25℃, 3 days. Image from [http://www.pf.chiba-u.ac.jp/gallery/fungi/c/Conidiobolus_coronatus_colony1.htm Chiba University Research Center for Pathogenic Fungi and Microbial Toxicoses]]] | ||
C. coronatus has wide vegetative coenocytic hyphae that are 6 to 15 µm in size. The conidiophores are 8-12 µm in width by 60-90 µm in length and they produce primary conidia that are 25 to 45 µm in diameter, spherical, single-celled and have a nipple-like | |||
''C. coronatus'' grows on potato dextrose, Sabouraud and cornmeal agar. The strains that are adapted to human infection readily grow at 37°C. Colonies of ''C. coronatus'' are fast growing, flat, waxy, creamy white to tan with age. As a colony becomes older it obtains powdery texture and the lid of the petri dish becomes covered with sticky conidia. The colonies grow quickly and can reach a diameter of 6 cm in 48 hours. The species are easy to maintain in the laboratory environment. | |||
C. coronatus has wide vegetative coenocytic hyphae that are 6 to 15 µm in size. The conidiophores are 8-12 µm in width by 60-90 µm in length and they produce primary conidia that are 25 to 45 µm in diameter, spherical, single-celled and have a nipple-like structure at the base that assists in discharging conidia. The villose conidia’s spikes are 10 to 15 µm in length. | |||
==Life cycle and metabolism == | ==Life cycle and metabolism == | ||
C. coronatus is free-living, not obligate intracellular parasite. | ''C. coronatus'' is free-living, not obligate intracellular parasite. | ||
It | It has complex asexual life cycle. When primary conidia are produced from the hyphae, the columella projects into the conidia. The nipple-like structure at the base of the conidia releases the primary conidia up to 4 cm into the air using the evertion mechanism which is the characteristic feature of the ''Conidiobolus'' genus <sup>2</sup>. | ||
Spherical conidia with hair-like appendages (villae) and prominent papillae characteristic of C. coronatus | Spherical conidia with hair-like appendages (villae) and prominent papillae characteristic of C. coronatus | ||
The primary conidia can germinate by forming hyphae from multiple germ tubes or give rise to secondary conidia that are oriented by light and are also released by eversion | The primary conidia can germinate by forming hyphae from multiple germ tubes or give rise to secondary conidia that are oriented by light and are also released by eversion <sup>3</sup>. ''C. coronatus'' also produces several other forms of secondary spores such as microconidia, chlamydospores and locriconidia. ''C. coronatus'' is unique to its genus because it produces villose conidia, from which it gets its name because the spikes look like a crown. | ||
Secondary conidia production is regulated by the availability of nutrients and pH. Nutrient rich or mildly acidic or basic medium produces germination of germ tubes to form hyphae, while nutrient poor or extremely acidic and basic medium will lead to the production of secondary conidia. Younger cultures and cultures in humid environments tend to produce the microconidia, while older cultures tend to produce the villose conidia. | Secondary conidia production is regulated by the availability of nutrients and pH. Nutrient rich or mildly acidic or basic medium produces germination of germ tubes to form hyphae, while nutrient poor or extremely acidic and basic medium will lead to the production of secondary conidia. Younger cultures and cultures in humid environments tend to produce the microconidia, while older cultures tend to produce the villose conidia. | ||
[http://genome.jgi.doe.gov/cgi-bin/metapathways?db=Conco1&batchId=Conco1:1 Bioinformatical models of ''C. coronatus'' metabolic pathways] | |||
==Ecology== | ==Ecology== | ||
C. coronatus has a world-wide distribution. It is the most commonly isolated species of the Conidiobolus genus. | ''C. coronatus'' has a world-wide distribution. It is the most commonly isolated species of the ''Conidiobolus'' genus. It prefers warmer, wetter, tropical and subtropical climates around the equator. | ||
==Pathology== | ==Pathology== | ||
Only about | [[File: Infectedlarvae.jpg|300px|thumb|left| ''Galleria mellonella larvae infected with Conidiobolus coronatus'' Image from [http://www.ipar.pan.pl/?module=lab&lab=lab23&language=en Laboratory of Physiology, Institute of Parasitology, Polish Academy of Sciences]]] | ||
Rhinofacial entomophthoromycosis caused by C. coronatus results from the inhalation of fungal spores that then infect nasal mucosa and from there can spread throughout the subcutaneous of the face paranasal sinuses and even brain. The lesions are painless and form nodules throughout the face of the infected person. These nodules heavily deform individual’s appearance. If there is swelling inside the nasal cavity, it can lead to the sensation of nasal obstruction. The serine proteases are excreted into the subcutaneous space, where they cause the release of amino acids by degrading proteins. However, the | |||
[[File: humancc.jpg|200px|thumb|right| ''Facial feature of the patient with conidiobolomycosis caused by ''Conidiobolus coronatus'' Image from [http://onlinelibrary.wiley.com/doi/10.1111/j.1439-0507.2009.01716.x/full Yang, X., Li, Y., Zhou, X., Wang, Y., Geng, S., Liu, H., Yang, Q., Lu, X., Hiruma, M., Sugita, T., Ikeda, S. and Ogawa, H. (2010), Rhinofacial conidiobolomycosis caused by Conidiobolus coronatus in a Chinese rice farmer. ''Mycoses'', 53: 369–373. doi: 10.1111/j.1439-0507.2009.01716.x]]] | |||
''C. coronatus'' parasitizes on many insects. It secrets protein-degrading enzymes that are able to degrade the insect cuticle which makes it the most important virulence factor of the fungi <sup>4</sup>. The excretion of serine proteases that are produced when the conidia are released is the most important virulence factor of C. coronatus. This mode of virulence is postulated because there is an increase in the production of serine proteases during columella and conidia formation. C coronatus also produces tissue destroying collagenases and lipases. It spreads throughout the body, eventually rupturing the exoskeleton to sporulate. Direct cause of insect host death is an effect of mycotoxins that damage the tissues. After killing the host, ''C. coronatus'' develops saprophytically. | |||
Only about 170 cases of human disease have been observed. The first human infection case on the Grand Cayman Islands was described by Bras et al in 1965 <sup>5</sup>. The fungus usually infects men that are agriculture or outdoor workers between the ages of 20-60. The individuals that are infected are often vulnerable to opportunistic infections, but in many cases the patients have been healthy with no physical abnormalities. The majority of the human infections occur in Nigeria, Cameroon, the Ivory Coast and Zaire. | |||
Rhinofacial entomophthoromycosis caused by ''C. coronatus'' results from the inhalation of fungal spores that then infect nasal mucosa and from there can spread throughout the subcutaneous of the face paranasal sinuses and even brain. The lesions are painless and form nodules throughout the face of the infected person. These painless nodules heavily deform individual’s appearance. If there is swelling inside the nasal cavity, it can lead to the sensation of nasal obstruction. The serine proteases are excreted into the subcutaneous space, where they cause the release of amino acids by degrading proteins. However, the fungus rarely passes the blood-brain barrier or into the lungs and rarely cause a human death <sup>6</sup>. The majority of the human infections occur in Western African rain forests in Nigeria, Cameroon, the Ivory Coast and Zaire. Infections also have been documented in India, Colombia, Brazil, Jamaica, Coast Rica, the Republic of Congo, China and Japan. | |||
Due to the rarity of the infection, diagnosis is usually established late. There is no standard regimen for treatment of conidiobolomycosis. | |||
==References== | |||
<sup>1</sup> [http://www.jstor.org/stable/3756277 Emmons C.W. and Bridges C.H. (1961) Entomophthora coronata, the Etiologic Agent of a Phycomycosis of Horses. <i>Mycologia</i>. 53(3): 307-312] | |||
<sup>2</sup> Lacey J. (1986) <b>Water availability and fungal reproduction: patterns of spore production, liberation and dispersal In: Water, Fungi and Plants</b> (eds. Ayres P.G., Boddy L.) Cambridge University Press, Cambridge. pp. 65-86 | |||
<sup>3</sup> [http://www.jstor.org/stable/3758106 Page R.M. and Humber R.A. (1973) Phototropism in Conidiobolus coronatus. <i>Mycologia</i>. 65(2): 335-354] | |||
<sup>4</sup> [http://dx.doi.org/10.1002/arch.20134 Bania, J., Samborski, J., Bogus, M. and Polanowski, A. (2006), Specificity of an extracellular proteinase from ''Conidiobolus coronatus'' and its inhibition by an inhibitor from insect hemolymph. ''Arch. Insect Biochem. Physiol.'', 62: 186–196. doi: 10.1002/arch.20134] | |||
<sup>5</sup> [http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=14248986 G. Bras, C. C. Gordon, C. W. Emmons, K. M. Prendegast, and M. Sugar (1965) A Case of Phycomycosis Observed in Jamaica; Infection with Entomophthora Coronata. <i>Am J Trop Med Hyg</i> 14:141-145] | |||
<sup>6</sup> [http://www.ncbi.nlm.nih.gov/pubmed/22728716 Walker SD, Clark RV, King CT, Humphries JE, Lytle LS, Butkus DE. (1992) Fatal disseminated Conidiobolus coronatus infection in a renal transplant patient. <i>Am J Clin Pathol</i> 98(6):559-64] | |||
Latest revision as of 15:18, 22 February 2016
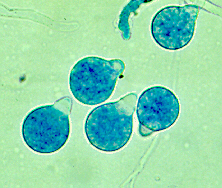
Classification
Higher order taxa
Kingdom: Eukaryota
Domain: Fungi
Phylum: Entomophthoromycota
Subphylum: Entomophthoramycotina
Order: Entomophthorales
Family: Ancylistaceae
Genus: Conidiobolus
Species
Conidiobolus coronatus
Synonym: Entomophthora coronata, Delacroixia coronata
|
NCBI: Taxonomy |
Description and significance
C. coronatus was first described by Constantin in 1897 in France and first isolated by Emmons and Bridges in 1961 in Australia 1. It is commonly present in soil and decaying organic matter and has a world-wide distribution with a high prevalence in tropical and subtropical regions. C. coronatus is the major causative agent of rare condition of rhinofacial entomophthoromycosis (conidiobolomycosis) in humans and other mammals, such as horses, dogs and llamas. It is also known to parasitize large variety of insects by rupturing the exoskeleton and killing the insect host within 1-2 days.
Genome structure
|
NCBI: Genome |
The DOE Joint Genome Institute has sequenced draft assembly of C. coronatus NRRL28638 strain genome using 454 technology. The genome of contains 39,90 Mbp and has been partially annotated. Total gene count - 6929 genes.
Cell and colony structure
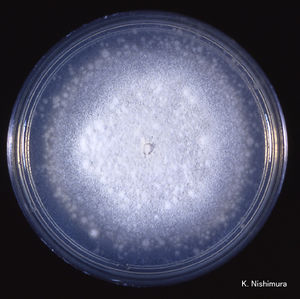
C. coronatus grows on potato dextrose, Sabouraud and cornmeal agar. The strains that are adapted to human infection readily grow at 37°C. Colonies of C. coronatus are fast growing, flat, waxy, creamy white to tan with age. As a colony becomes older it obtains powdery texture and the lid of the petri dish becomes covered with sticky conidia. The colonies grow quickly and can reach a diameter of 6 cm in 48 hours. The species are easy to maintain in the laboratory environment. C. coronatus has wide vegetative coenocytic hyphae that are 6 to 15 µm in size. The conidiophores are 8-12 µm in width by 60-90 µm in length and they produce primary conidia that are 25 to 45 µm in diameter, spherical, single-celled and have a nipple-like structure at the base that assists in discharging conidia. The villose conidia’s spikes are 10 to 15 µm in length.
Life cycle and metabolism
C. coronatus is free-living, not obligate intracellular parasite. It has complex asexual life cycle. When primary conidia are produced from the hyphae, the columella projects into the conidia. The nipple-like structure at the base of the conidia releases the primary conidia up to 4 cm into the air using the evertion mechanism which is the characteristic feature of the Conidiobolus genus 2. Spherical conidia with hair-like appendages (villae) and prominent papillae characteristic of C. coronatus The primary conidia can germinate by forming hyphae from multiple germ tubes or give rise to secondary conidia that are oriented by light and are also released by eversion 3. C. coronatus also produces several other forms of secondary spores such as microconidia, chlamydospores and locriconidia. C. coronatus is unique to its genus because it produces villose conidia, from which it gets its name because the spikes look like a crown.
Secondary conidia production is regulated by the availability of nutrients and pH. Nutrient rich or mildly acidic or basic medium produces germination of germ tubes to form hyphae, while nutrient poor or extremely acidic and basic medium will lead to the production of secondary conidia. Younger cultures and cultures in humid environments tend to produce the microconidia, while older cultures tend to produce the villose conidia.
Bioinformatical models of C. coronatus metabolic pathways
Ecology
C. coronatus has a world-wide distribution. It is the most commonly isolated species of the Conidiobolus genus. It prefers warmer, wetter, tropical and subtropical climates around the equator.
Pathology

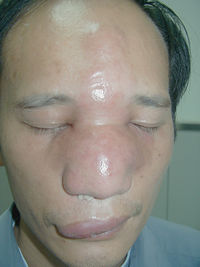
C. coronatus parasitizes on many insects. It secrets protein-degrading enzymes that are able to degrade the insect cuticle which makes it the most important virulence factor of the fungi 4. The excretion of serine proteases that are produced when the conidia are released is the most important virulence factor of C. coronatus. This mode of virulence is postulated because there is an increase in the production of serine proteases during columella and conidia formation. C coronatus also produces tissue destroying collagenases and lipases. It spreads throughout the body, eventually rupturing the exoskeleton to sporulate. Direct cause of insect host death is an effect of mycotoxins that damage the tissues. After killing the host, C. coronatus develops saprophytically.
Only about 170 cases of human disease have been observed. The first human infection case on the Grand Cayman Islands was described by Bras et al in 1965 5. The fungus usually infects men that are agriculture or outdoor workers between the ages of 20-60. The individuals that are infected are often vulnerable to opportunistic infections, but in many cases the patients have been healthy with no physical abnormalities. The majority of the human infections occur in Nigeria, Cameroon, the Ivory Coast and Zaire.
Rhinofacial entomophthoromycosis caused by C. coronatus results from the inhalation of fungal spores that then infect nasal mucosa and from there can spread throughout the subcutaneous of the face paranasal sinuses and even brain. The lesions are painless and form nodules throughout the face of the infected person. These painless nodules heavily deform individual’s appearance. If there is swelling inside the nasal cavity, it can lead to the sensation of nasal obstruction. The serine proteases are excreted into the subcutaneous space, where they cause the release of amino acids by degrading proteins. However, the fungus rarely passes the blood-brain barrier or into the lungs and rarely cause a human death 6. The majority of the human infections occur in Western African rain forests in Nigeria, Cameroon, the Ivory Coast and Zaire. Infections also have been documented in India, Colombia, Brazil, Jamaica, Coast Rica, the Republic of Congo, China and Japan.
Due to the rarity of the infection, diagnosis is usually established late. There is no standard regimen for treatment of conidiobolomycosis.
References
2 Lacey J. (1986) Water availability and fungal reproduction: patterns of spore production, liberation and dispersal In: Water, Fungi and Plants (eds. Ayres P.G., Boddy L.) Cambridge University Press, Cambridge. pp. 65-86
3 Page R.M. and Humber R.A. (1973) Phototropism in Conidiobolus coronatus. Mycologia. 65(2): 335-354
