Cryptococcosis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
Cryptococcosis is a life threatening fungal infection caused by members of the Cryptococcus genus. The two species known to cause cryptococcosis are C. neoformans and C. gattii. Cryptococcosis generally presents as one of three different infections, although all three infections can present in the same patient. Cutaneous cryptococcosis is an external infection, generally presenting as a rash or in an open wound. Pulmonary cryptococcosis is a lung infection, contracted by inhaling propagules formed by the adult fungi. Cryptococcal meningitis is an infection of the meninges in the brain, and is thought to result from the spread of the fungus in advanced cases of pulmonary cryptococcosis. Cryptococcosis is one of the most common AIDS-defining opportunistic infections in the world, and is rarely seen in non-immunocompromised persons <ref>[https://journals.lww.com/aidsonline/fulltext/2009/02200/Estimation_of_the_current_global_burden_of.12.aspx]</ref> | Cryptococcosis is a life threatening fungal infection caused by members of the Cryptococcus genus. The two species known to cause cryptococcosis are <i>C. neoformans</i> and <i>C. gattii</i>. Cryptococcosis generally presents as one of three different infections, although all three infections can present in the same patient. Cutaneous cryptococcosis is an external infection, generally presenting as a rash or in an open wound. Pulmonary cryptococcosis is a lung infection, contracted by inhaling propagules formed by the adult fungi. Cryptococcal meningitis is an infection of the meninges in the brain, and is thought to result from the spread of the fungus in advanced cases of pulmonary cryptococcosis. Cryptococcosis is one of the most common AIDS-defining opportunistic infections in the world, and is rarely seen in non-immunocompromised persons <ref>[https://journals.lww.com/aidsonline/fulltext/2009/02200/Estimation_of_the_current_global_burden_of.12.aspx Park et al.: "Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS" 2009. AIDS 23:4]</ref> | ||
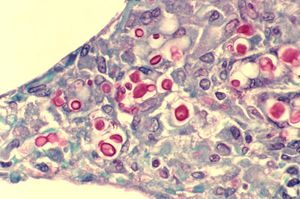
[[Image:Cryptococcosis_of_lung_in_patient_with_AIDS._Mucicarmine_stain_962_lores.jpg|thumb|300px|right|Cryptococcosis of the lung in a patient with AIDS. | [[Image:Cryptococcosis_of_lung_in_patient_with_AIDS._Mucicarmine_stain_962_lores.jpg|thumb|300px|right|Cryptococcosis of the lung in a patient with AIDS. | ||
The slide shows widened alveoli, with multiple yeasts of C. neoformans, stained red. Image courtesy of CDC/ Dr. Edwin P. Ewing, Jr. | The slide shows widened alveoli, with multiple yeasts of <i>C. neoformans</i>, stained red. Image courtesy of CDC/ Dr. Edwin P. Ewing, Jr. | ||
https://phil.cdc.gov/Details.aspx?pid=962]] | https://phil.cdc.gov/Details.aspx?pid=962]] | ||
<br> <br> | |||
==Section 1 Genetics== | |||
Genetic analysis and genome sequencing of <i>C. neoformans</i> and <i>C. gattii</i> is an important part of diagnosis and treatment of cryptococcosis. Multiple Locus Sequence Typing (MLST) analysis has been used to great effect, and of human pathogenic fungi these two species of Cryptococcus are some of the best mapped. <br><br> | |||
There are several issues that arise when trying to use genetic research for diagnosis and treatment in a clinical setting. The first is that these cryptococcal genomes show tremendous plasticity, and thus the ability to undergo microevolution. This has been observed in vivo in human patients, and presents a challenge to physicians attempting to treat patients with cryptococcosis. Even within strains isolated from patients, there has been observed minute genetic differences. Only through detailed mapping of the entire genome of both species and fully understanding and observing the genetic controls that allows this can this issue be overcome. <br><br> | |||
Another issue that arises is that <i>C. neoformans</i> and <i>C. gattii</i> are genetically different species and react differently to the same environmental stresses. This is to be expected, as they are different species, but it makes it difficult for the clinical treatment of patients. | |||
The two species also show a remarkable disconnect in their reactions in vivo and in vitro. For example, in vivo there has been shown resistance to fluconazole (a common antifungal medication) through gene plasticity and subsequent gene duplications for efflux pumps in both species. This contrasts with the reactions of the species in vitro, where if a previously resistant specimen is isolated and again exposed to fluconazole, it is fully susceptible. Obviously, this presents a challenge to the clinical treatment of cryptococcosis. <br><br> | |||
As of right now, genetic research into these two species of Cryptococcus is not fully applicable to clinical situations. There are techniques available to determine which species of Cryptococcus is causing the infection (CGB agar plate assays and MALDI-TOF are two common ones), but this information is not necessarily useful to the physician when determining a treatment course. Recent studies are beginning to link genetic variations in human immunity genes to increased susceptibility, which makes determining risk factors more difficult for physicians. <ref>[https://www.sciencedirect.com/science/article/pii/S1087184514001868 Perfect et al.: "Cryptococcosis diagnosis and treatment: What do we know now" 2015. Fungal Genetics and Biology 78]</ref> <br><br> | |||
<b><i>C. neoformans</i></b> <br> | |||
<i>C. neoformans</i> is the better studied of the two Cryptococcus species known to cause cryptococcosis. It was previously believed that C. gattii was merely a subspecies of C. neoformans, but it has now been recognized as a distinct species in its own right. <i>C. neoformans</i> is not particularly well adapted as a human pathogen. It does not have specific genetic survival mechanisms for human hosts, hence why infection is usually only seen in immunocompromised patients or in those with certain immune deficiencies. <br><br> | |||
<i>C. neoformans</i> has several adaptations that make it unique when compared to other human pathogens, especially pathogenic fungi. One of these is the formation of microvesicles. The pathogen uses these to transport virulence structures from interior synthesis sites to the extracellular matrix, where they will be utilized. These microvesicles have shown the ability to interact with the blood-brain barrier, which may be a factor that influences the ability of <i>C. neoformans</i> to infect the central nervous system. <i>C. neoformans</i> is also able to actively remodel its cell wall in response to host signals. This is a form of immune system evasion by hiding immunogenic features. <ref>[https://www.sciencedirect.com/science/article/pii/S1087184514001613 J. Andrew Alspaugh "Virulence mechanisms and <i>Cryptococcus neoformans</i> pathogenesis" 2015. Fungal Genetics and Biology 78]</ref> <br><br> | |||
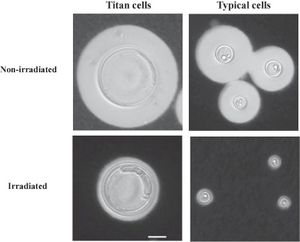
Under large amounts of stress from the host, <i>C. neoformans</i> has the ability to form extremely large cells (20-100 uM), which are largely immune to host responses. These cells, most commonly seen in pulmonary infections, have the ability to survive host responses and then repropagate the infection, and due to their polyploid nature can give rise to large amounts of genetic diversity. <ref>[https://www.sciencedirect.com/science/article/pii/S2468233017300634 Mukaremera et al.: "Titan cell production in <i>Cryptococcus neoformans</i> reshapes the cell wall and capsule composition during infection" 2018. The Cell Surface 1]</ref> <br><br> | |||
[[Image:1-s2.0-S2468233017300634-gr4.jpg|thumb|300px|left| Titan cells of <i>C. neoformans</i> shown next to typical <i>C. neoformans</i> cells . Image courtesy of L. Mukaremera | |||
https://www.sciencedirect.com/science/article/pii/S2468233017300634]] | |||
<br><br> | |||
==Section 2 Microbiome== | ==Section 2 Microbiome== | ||
Cryptococcosis is closely related with HIV/AIDS in human patients. It is an opportunistic infection, meaning that it is seen in these patients primarily because of their compromised immune system. Immunocompromisation is also a factor in cryptococcosis infections in organ donor patients. For a time, it was believed that this infection could only target immunocompromised patients, but this was later refuted. T-cell responses are vital to controlling cryptococcal infections, which is why it is a difficult infection to treat in immunocompromised patients.<ref>[https://www.sciencedirect.com/science/article/pii/S1087184514002151 Gibson et al.: "Immunity to <i>Cryptococcus neoformans</i> and <i>C. gattii</i> during cryptococcosis" 2015. Fungal Genetics and Biology 78]</ref> <br><br> | |||
<i>C. neoformans</i> and <i>C. gattii</i> are not normally known to damage host cells, but a mutant, <i>rim101</i>, has an altered cell surface that triggers different cytokines. These cytokines induce a massive inflammatory response that is damaging to inflammatory cells.<br><br> | |||
==Conclusion== | ==Conclusion== | ||
Cryptococcosis continues to be a massive public health issue, with an estimated 625,000 deaths per year. The outcome for HIV positive patients continues to get better year after year, but concerningly the outcome for patients considered “otherwise healthy” has not changed since the early days of the HIV epidemic. Estimates of US mortality predict that 80% of the deaths that have occured between the years of 2002-2013 were not HIV positive. This is most likely due to the delayed diagnosis in patients without HIV. Cryptococcosis is a defining infection within the HIV+/AIDS community, meaning that detection and diagnosis is generally fairly rapid within these populations. Outside of these groups, detection rates are lower, and thus the mortality rate is higher. More research will help to increase the detection and survival rates in all patients with cryptococcosis. <ref>[https://www.sciencedirect.com/science/article/pii/S1087184515000985 Idnurm et al.: "Rising to the challenge of multiple <i>Cryptococcus</i> species and the diseases they cause" 2015. Fungal Genetics and Biology 78]</ref> <br><br> | |||
==References== | ==References== | ||
< | Park et al.: "Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS" 2009. AIDS 23:4 <br> | ||
Perfect et al.: "Cryptococcosis diagnosis and treatment: What do we know now" 2015. Fungal Genetics and Biology 78 <br> | |||
J. Andrew Alspaugh "Virulence mechanisms and <i>Cryptococcus neoformans</i> pathogenesis" 2015. Fungal Genetics and Biology 78 <br> | |||
Mukaremera et al.: "Titan cell production in <i>Cryptococcus neoformans</i> reshapes the cell wall and capsule composition during infection" 2018. The Cell Surface 1 <br> | |||
Gibson et al.: "Immunity to <i>Cryptococcus neoformans</i> and <i>C. gattii</i> during cryptococcosis" 2015. Fungal Genetics and Biology 78 <br> | |||
Idnurm et al.: "Rising to the challenge of multiple <i>Cryptococcus</i> species and the diseases they cause" 2015. Fungal Genetics and Biology 78 <br> | |||
<br>Edited by Eli Neal, student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol116/biol116_Fall_2013.html BIOL 116 Information in Living Systems], 2019, [http://www.kenyon.edu/index.xml Kenyon College]. | <br>Edited by Eli Neal, student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol116/biol116_Fall_2013.html BIOL 116 Information in Living Systems], 2019, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Revision as of 05:47, 6 December 2019
Introduction
Cryptococcosis is a life threatening fungal infection caused by members of the Cryptococcus genus. The two species known to cause cryptococcosis are C. neoformans and C. gattii. Cryptococcosis generally presents as one of three different infections, although all three infections can present in the same patient. Cutaneous cryptococcosis is an external infection, generally presenting as a rash or in an open wound. Pulmonary cryptococcosis is a lung infection, contracted by inhaling propagules formed by the adult fungi. Cryptococcal meningitis is an infection of the meninges in the brain, and is thought to result from the spread of the fungus in advanced cases of pulmonary cryptococcosis. Cryptococcosis is one of the most common AIDS-defining opportunistic infections in the world, and is rarely seen in non-immunocompromised persons [1]
Section 1 Genetics
Genetic analysis and genome sequencing of C. neoformans and C. gattii is an important part of diagnosis and treatment of cryptococcosis. Multiple Locus Sequence Typing (MLST) analysis has been used to great effect, and of human pathogenic fungi these two species of Cryptococcus are some of the best mapped.
There are several issues that arise when trying to use genetic research for diagnosis and treatment in a clinical setting. The first is that these cryptococcal genomes show tremendous plasticity, and thus the ability to undergo microevolution. This has been observed in vivo in human patients, and presents a challenge to physicians attempting to treat patients with cryptococcosis. Even within strains isolated from patients, there has been observed minute genetic differences. Only through detailed mapping of the entire genome of both species and fully understanding and observing the genetic controls that allows this can this issue be overcome.
Another issue that arises is that C. neoformans and C. gattii are genetically different species and react differently to the same environmental stresses. This is to be expected, as they are different species, but it makes it difficult for the clinical treatment of patients.
The two species also show a remarkable disconnect in their reactions in vivo and in vitro. For example, in vivo there has been shown resistance to fluconazole (a common antifungal medication) through gene plasticity and subsequent gene duplications for efflux pumps in both species. This contrasts with the reactions of the species in vitro, where if a previously resistant specimen is isolated and again exposed to fluconazole, it is fully susceptible. Obviously, this presents a challenge to the clinical treatment of cryptococcosis.
As of right now, genetic research into these two species of Cryptococcus is not fully applicable to clinical situations. There are techniques available to determine which species of Cryptococcus is causing the infection (CGB agar plate assays and MALDI-TOF are two common ones), but this information is not necessarily useful to the physician when determining a treatment course. Recent studies are beginning to link genetic variations in human immunity genes to increased susceptibility, which makes determining risk factors more difficult for physicians. [2]
C. neoformans
C. neoformans is the better studied of the two Cryptococcus species known to cause cryptococcosis. It was previously believed that C. gattii was merely a subspecies of C. neoformans, but it has now been recognized as a distinct species in its own right. C. neoformans is not particularly well adapted as a human pathogen. It does not have specific genetic survival mechanisms for human hosts, hence why infection is usually only seen in immunocompromised patients or in those with certain immune deficiencies.
C. neoformans has several adaptations that make it unique when compared to other human pathogens, especially pathogenic fungi. One of these is the formation of microvesicles. The pathogen uses these to transport virulence structures from interior synthesis sites to the extracellular matrix, where they will be utilized. These microvesicles have shown the ability to interact with the blood-brain barrier, which may be a factor that influences the ability of C. neoformans to infect the central nervous system. C. neoformans is also able to actively remodel its cell wall in response to host signals. This is a form of immune system evasion by hiding immunogenic features. [3]
Under large amounts of stress from the host, C. neoformans has the ability to form extremely large cells (20-100 uM), which are largely immune to host responses. These cells, most commonly seen in pulmonary infections, have the ability to survive host responses and then repropagate the infection, and due to their polyploid nature can give rise to large amounts of genetic diversity. [4]
Section 2 Microbiome
Cryptococcosis is closely related with HIV/AIDS in human patients. It is an opportunistic infection, meaning that it is seen in these patients primarily because of their compromised immune system. Immunocompromisation is also a factor in cryptococcosis infections in organ donor patients. For a time, it was believed that this infection could only target immunocompromised patients, but this was later refuted. T-cell responses are vital to controlling cryptococcal infections, which is why it is a difficult infection to treat in immunocompromised patients.[5]
C. neoformans and C. gattii are not normally known to damage host cells, but a mutant, rim101, has an altered cell surface that triggers different cytokines. These cytokines induce a massive inflammatory response that is damaging to inflammatory cells.
Conclusion
Cryptococcosis continues to be a massive public health issue, with an estimated 625,000 deaths per year. The outcome for HIV positive patients continues to get better year after year, but concerningly the outcome for patients considered “otherwise healthy” has not changed since the early days of the HIV epidemic. Estimates of US mortality predict that 80% of the deaths that have occured between the years of 2002-2013 were not HIV positive. This is most likely due to the delayed diagnosis in patients without HIV. Cryptococcosis is a defining infection within the HIV+/AIDS community, meaning that detection and diagnosis is generally fairly rapid within these populations. Outside of these groups, detection rates are lower, and thus the mortality rate is higher. More research will help to increase the detection and survival rates in all patients with cryptococcosis. [6]
References
Park et al.: "Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS" 2009. AIDS 23:4
Perfect et al.: "Cryptococcosis diagnosis and treatment: What do we know now" 2015. Fungal Genetics and Biology 78
J. Andrew Alspaugh "Virulence mechanisms and Cryptococcus neoformans pathogenesis" 2015. Fungal Genetics and Biology 78
Mukaremera et al.: "Titan cell production in Cryptococcus neoformans reshapes the cell wall and capsule composition during infection" 2018. The Cell Surface 1
Gibson et al.: "Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis" 2015. Fungal Genetics and Biology 78
Idnurm et al.: "Rising to the challenge of multiple Cryptococcus species and the diseases they cause" 2015. Fungal Genetics and Biology 78
Edited by Eli Neal, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2019, Kenyon College.
- ↑ Park et al.: "Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS" 2009. AIDS 23:4
- ↑ Perfect et al.: "Cryptococcosis diagnosis and treatment: What do we know now" 2015. Fungal Genetics and Biology 78
- ↑ J. Andrew Alspaugh "Virulence mechanisms and Cryptococcus neoformans pathogenesis" 2015. Fungal Genetics and Biology 78
- ↑ Mukaremera et al.: "Titan cell production in Cryptococcus neoformans reshapes the cell wall and capsule composition during infection" 2018. The Cell Surface 1
- ↑ Gibson et al.: "Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis" 2015. Fungal Genetics and Biology 78
- ↑ Idnurm et al.: "Rising to the challenge of multiple Cryptococcus species and the diseases they cause" 2015. Fungal Genetics and Biology 78
