Cucurbit yellow stunting disorder virus (CYSDV): Difference between revisions
| Line 26: | Line 26: | ||
<br> | <br> | ||
<br> | <br> | ||
RNA1 is 9123 nt long and contains at least five open reading frames (ORFs) [5]. RNA2 is 7976 nt long and contains the gene array distinctive to the family Closteroviridae, | RNA1 is 9123 nt long and contains at least five open reading frames (ORFs) [5]. RNA2 is 7976 nt long and contains the gene array distinctive to the family <i>Closteroviridae</i>, | ||
characterized by ORFs encoding a heat shock protein 70 homologue (HSP70h), two proteins of unknown function (58 and p9), the major coat protein (CP) and a divergent copy of the coat protein (CPm) [2] [4] [5]. | characterized by ORFs encoding a heat shock protein 70 homologue (HSP70h), two proteins of unknown function (58 and p9), the major coat protein (CP) and a divergent copy of the coat protein (CPm) [2] [4] [5]. | ||
Revision as of 01:51, 10 September 2010
A Viral Biorealm page on the family Cucurbit yellow stunting disorder virus (CYSDV)

Baltimore Classification
Group IV: (+) sense single-stranded RNA viruses
Higher order categories
Order: Unassigned
Family: Closteroviridae
Genus: Crinivirus
Description and Significance
Cucurbit yellow stunting disorder virus, CYSDV, can infect members of the family Cucurbitaceae, including all types of melons, summer and winter squash, pumpkins, gourds, and cucumbers [1]. CYSDV symptoms develop first in older leaves and mimic water stress. Intervenial chlorosis, a yellowing between the veins, streaks the leaves. Eventually the entire leaf becomes yellow except for the veins, which remain green [2]. In certain varieties, small green spots may develop on the leaves as well. As the plant’s internal transport system breaks down, it begins to drop older leaves in attempt to preserve itself. Without enough leaves, the plant’s strength dwindles and it no longer can support or nourish its fruit. As a result, the fruits are smaller, not as sweet, and don’t ship or store as well [1]. Consequently, CYSDV has significant economic implications on national and international agriculture. However, control is difficult because there is no chemical or biological control suitable to fight the virus. Increasing water and fertilizer, and early season insecticide application (to reduce the virus’s vector population) may help, but these remedies compromise the producer’s ability to grow a sustainable crop and are incredibly costly [1].
Genome Structure
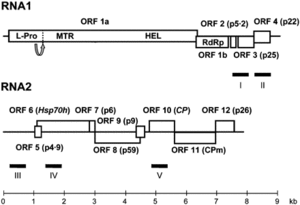
The CYSDV genome consists of two molecules of (+) sense ssRNA designated RNA1 and RNA2.
Although over seventy isolates of CYSDV have been identified around the world, in countries including Spain, Jordan, Turkey, Lebanon, Saudi Arabia, and North America, two genetically distinct subpopulations have been identified. The two subpopulations are the so-called Eastern subpopulation, composed of the Saudia Arabian isolates and the Western subpopulation, containing the rest of the CYSDV isolates [2] [4]. The genomes of many of the isolates have yet to be sequenced. However, the complete nucleotide (nt) sequences of genomic RNAs 1 and 2 of for the Spanish isolate CYSDV-AlLM are known.
RNA1 is 9123 nt long and contains at least five open reading frames (ORFs) [5]. RNA2 is 7976 nt long and contains the gene array distinctive to the family Closteroviridae,
characterized by ORFs encoding a heat shock protein 70 homologue (HSP70h), two proteins of unknown function (58 and p9), the major coat protein (CP) and a divergent copy of the coat protein (CPm) [2] [4] [5].
Virion Structure of CYSDV
CYSDV virus particles are flexous rods 750-800 nm in length. The virus ecapsidates 2 molecules of (+)sense ssRNA, designated RNA1 and RNA2 [2].
Reproductive Cycle of CYSDV in a Host Cell
Virus penetrates into the host cell.
Uncoating, and release of the viral genomic RNA into the cytoplasm.
The viral RNA is translated.
Negative-sense complementary ssRNAs are synthesized using the genomic RNAs as a templates.
New genomic RNAs are synthesized using the negative-sense RNAs as templates.
Formation of new virus particles [3].
Viral Ecology & Pathology
References
[1] McGinley, Susan. 2010. "New virus attacks melons, cucumbers, and squash." Western Farm Press. http://westernfarmpress.com/new-virus-attacks-melons-cucumbers-and-squash-0. Date accessed: 6 Sept. 2010.
[2] Auguilar, Juan M. et al. Resistance to Cucurbit yellow stunting disorder virus in Cucumber. Plant Diseases. 90.5 (2006): 583.
[3] "Viral Zone: Closteroviridae". ExPASY Proteomics Server. http://www.expasy.org/viralzone/all_by_species/34.html.
[4] Rubio, Luis et al. Geographically distant isolates of the crinivirus Cucurbit yellow stunting disorder virus show very low genetic diversity in the coat protein gene. Journal of General Virology. 82 (2001): 929.
[5] Augilar, Juan M. et al. Further variability within the genus Crinivirus, as revealed by determination of the complete RNA genome sequence of Cucurbit yellow stunting disorder virus. Journal of General Virology. 84 (2003): 2555.
Page authored by Sally Wilson for BIOL 375 Virology, September 2010
