Cysticercosis: Difference between revisions
No edit summary |
|||
| (35 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Conway}} | |||
{{ | [[File:Taenia solium.JPEG|700px|thumb|right|Image of <i>Taenia solium</i>. From: faculty.ccbcmd.edu [http://faculty.ccbcmd.edu/courses/bio141/labmanua/lab20/scolex.html]]] | ||
[[File:Taenia solium.JPEG| | |||
==Etiology/Bacteriology== | ==Etiology/Bacteriology== | ||
===Taxonomy=== | ===Taxonomy=== | ||
| Line 12: | Line 10: | ||
| Genus = [[Taenia]] | | Genus = [[Taenia]] | ||
| species = [[T. solium]] | | species = [[T. solium]] | ||
{| | |||
< | | height="10" bgcolor="#FFDF95" | | ||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=6204&lvl=3&keep=1&srchmode=1&unlock&lin=s Taxonomy] <font size="2"></font>''' | |||
|} | |||
===Description=== | ===Description=== | ||
| Line 22: | Line 22: | ||
==Pathogenesis== | ==Pathogenesis== | ||
===Transmission=== | ===Transmission=== | ||
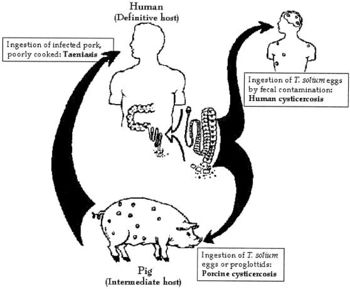
Larvae of the tapeworm <i>Taenia solium</i> are what cause cysticercosis. If a person becomes contaminated with an adult tapeworm, an infection called taeniasis, they can shed <i>T. solium</i> eggs in their stool. These eggs must then be consumed through fecal-oral route transmission, which can occur by drinking water or eating food that is contaminated with <i>T. solium</i> eggs, or by putting a fecal contaminated object in one’s mouth. Due to this form of transmission, it is possible for autoinfection to occur. If a person has taeniasis, they can infect themselves with the <i>T. solium</i> eggs they shed and thus develop cysticercosis. | Larvae of the tapeworm <i>Taenia solium</i> are what cause cysticercosis. If a person becomes contaminated with an adult tapeworm, an infection called taeniasis, they can shed <i>T. solium</i> eggs in their stool. These eggs must then be consumed through fecal-oral route transmission, which can occur by drinking water or eating food that is contaminated with <i>T. solium</i> eggs, or by putting a fecal contaminated object in one’s mouth. Due to this form of transmission, it is possible for autoinfection to occur. If a person has taeniasis, they can infect themselves with the <i>T. solium</i> eggs they shed and thus develop cysticercosis. [[#References|[1]]] | ||
===Infectious dose, incubation, and colonization=== | ===Infectious dose, incubation, and colonization=== | ||
The infectious dose required to cause cysticercosis is relatively unknown | The infectious dose required to cause cysticercosis is relatively unknown [[#References|[2]]]. The terminal end of the <i>T. solium</i> tapeworm consists of gravid proglottids, which are full of eggs. Upon the release of these eggs in fecal matter, a person is susceptible to consuming more than one egg. This increases the chances of developing cysticercosis. The median incubation period for cysticercosis is estimated to be about 3.5 years [[#References|[3]]] and can range from 10 days to 10 years [[#References|[4]]]. A person who has consumed <i>T. solium</i> eggs may go for years without having any symptoms of infection. This is due to the fact that cysticerci often only cause symptoms when they are dying or become large enough to interfere with vital organs. [[#References|[4]]] Furthermore, it normally takes 2-3 months for the eggs to develop into cysticerci [[#References|[2]]]. | ||
[[File:Life Cycle Picture.JPEG|350px|thumb|right|Life cycle of <i>Taenia solium</i>. From: NCIB [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC126865/]]] | [[File:Life Cycle Picture.JPEG|350px|thumb|right|Life cycle of <i>Taenia solium</i>. From: NCIB [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC126865/]]] | ||
Upon consumption of <i>T. solium</i> eggs, the oncospheres hatch in the intestine and then penetrate the intestinal wall. <i>T. solium</i> contain sharp, spine hooks and four suckers that help them adhere to and penetrate the gut wall | Upon consumption of <i>T. solium</i> eggs, the oncospheres hatch in the intestine and then penetrate the intestinal wall. <i>T. solium</i> contain sharp, spine hooks and four suckers that help them adhere to and penetrate the gut wall [[#References|[5]]]. They then migrate to skeletal muscle, the brain, eyes, subcutaneous tissues, and other places where they develop into cysticerci. [[#References|[6]]] | ||
===Epidemiology=== | ===Epidemiology=== | ||
<i>Taenia solium</i> is prevalent in parts of Africa, Asia, Mexico, and Latin America where humans live in close proximity to pigs. This is because pigs are one of the hosts during the life cycle of this two-host parasite, while humans are the other host. When pigs eat <i>T. solium</i> eggs, the eggs develop into larvae and form cysts within the pig’s muscles and other tissues. Then, if humans consume undercooked or raw infected pork, they swallow these cysts. The larvae then come out of their cysts within the human gut and develop into adult tapeworms. Now, this person will shed <i>T. solium</i> eggs in their stool. At this point, if fecal-oral contamination occurs, cysticercosis will develop in whoever consumed the contaminated fecal matter. | <i>Taenia solium</i> is prevalent in parts of Africa, Asia, Mexico, and Latin America where humans live in close proximity to pigs. This is because pigs are one of the hosts during the life cycle of this two-host parasite, while humans are the other host. When pigs eat <i>T. solium</i> eggs, the eggs develop into larvae and form cysts within the pig’s muscles and other tissues. Then, if humans consume undercooked or raw infected pork, they swallow these cysts. The larvae then come out of their cysts within the human gut and develop into adult tapeworms. Now, this person will shed <i>T. solium</i> eggs in their stool. At this point, if fecal-oral contamination occurs, cysticercosis will develop in whoever consumed the contaminated fecal matter. [[#References|[1]]] It is clear that cysticercosis is spread by fecal-oral route, not the consumption of undercooked, infected pork meat. | ||
===Virulence factors=== | ===Virulence factors=== | ||
<i>T. solium</i> contains a number of virulence factors that allow it to survive amidst the host immune response. The oncospheres invade the host and are susceptible to antibody and complement responses, but by the time the host actually mounts an antibody response, the parasite begins to transform to the more resistant larval stage, called metacestode. During the larval stage, the parasite can evade complement-mediated destruction. This is achieved by a few different virulence factors including paramyosin, which is a protein that inhibits C1q; taeniaestatin, a proteinase, inhibits the classical and alternate pathways; and sulfated polysaccharides, which activate complement away from the parasite. The larval stage of <i>T. solium</i> also manipulates antibody production in such a way that the host produces antibody that cannot kill the mature metacestode, but instead provides a protein source to the parasite. Lastly, taeniaestatin may interfere with lymphocyte proliferation and macrophage functions; therefore, compromising the cellular immune response. | <i>T. solium</i> contains a number of virulence factors that allow it to survive amidst the host immune response. The oncospheres invade the host and are susceptible to antibody and complement responses, but by the time the host actually mounts an antibody response, the parasite begins to transform to the more resistant larval stage, called metacestode. During the larval stage, the parasite can evade complement-mediated destruction. This is achieved by a few different virulence factors including paramyosin, which is a protein that inhibits C1q; taeniaestatin, a proteinase, inhibits the classical and alternate pathways; and sulfated polysaccharides, which activate complement away from the parasite. The larval stage of <i>T. solium</i> also manipulates antibody production in such a way that the host produces antibody that cannot kill the mature metacestode, but instead provides a protein source to the parasite. Lastly, taeniaestatin may interfere with lymphocyte proliferation and macrophage functions; therefore, compromising the cellular immune response. [[#References|[7]]] | ||
==Clinical features== | ==Clinical features== | ||
Cysticercosis is an infectious disease that causes the development of cysts, called cysticerci, in various locations of the body | Cysticercosis is an infectious disease that causes the development of cysts, called cysticerci, in various locations of the body [[#References|[8]]]. The most popular locations for the cysticerci are the subcutaneous tissues, skeletal muscle, eyes, brain, and spinal cord. The location of the cysts determines a lot about the symptoms, severity, and treatment of the disease. [[#References|[9]]] | ||
===Symptoms=== | ===Symptoms=== | ||
There are a variety of symptoms that can be demonstrated depending on the location and number of the cysts. If they are located in the skeletal muscle or subcutaneous tissue, there will often times be no signs of symptoms | There are a variety of symptoms that can be demonstrated depending on the location and number of the cysts. If they are located in the skeletal muscle or subcutaneous tissue, there will often times be no signs of symptoms [[#References|[8]]]. However, it is sometimes possible to feel lumps and palpable nodules beneath the skin, which may become tender and cause localized pain. If the cysts are located in the eye (usually in the subretinal space or vitreous humor), vision might become blurry and disturbed. This is due to inflammation or retinal detachment. [[#References|[9]]] Cysts in the brain or spinal cord cause a specific type of cysticercosis called neurocysticercosis. This is the most severe type of this infectious disease and causes the most threatening symptoms. Symptoms of neurocysticercosis include: headache, seizures, confusion, difficulty with balance, gait disturbances, brain swelling, hydrocephalus (excess fluid around the brain), stroke, and even death [[#References|[8]]]. In fact, cysticercosis is the single most common cause of acquired epileptic seizures [[#References|[10]]]. Obviously, the symptoms of this disease vary depending on the location of the cysts. | ||
===Morbidity and Mortality=== | ===Morbidity and Mortality=== | ||
[[File:Global distribution.png|300px|thumb|right|Global distribution of cysticercosis. From: WHO [http://www.who.int/taeniasis/Global_distribution_cysticercosis_2011.png?ua=1]]] | [[File:Global distribution.png|300px|thumb|right|Global distribution of cysticercosis. From: WHO [http://www.who.int/taeniasis/Global_distribution_cysticercosis_2011.png?ua=1]]] | ||
Cysticercosis is the most common parasitic disease across the world, and it is estimated that 50 million people are infected with it. The highest rates of infection for cysticercosis are found in parts of Africa, Asia, Mexico, and Central and South America. | Cysticercosis is the most common parasitic disease across the world, and it is estimated that 50 million people are infected with it. The highest rates of infection for cysticercosis are found in parts of Africa, Asia, Mexico, and Central and South America. [[#References|[9]]] However, it is becoming more of an issue in the United States because of increased immigration and world travelling. The listed locations tend to have poor sanitation and free-roaming pigs, which are environments that <i>Taenia solium</i> thrive and fecal-oral contamination can more easily occur. [[#References|[11]]] | ||
[[File:US Distribution.JPEG|300px|thumb|left|Fatal cysticercosis cases by state 1990-2002. From: NCBI [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2725874/]]] | [[File:US Distribution.JPEG|300px|thumb|left|Fatal cysticercosis cases by state 1990-2002. From: NCBI [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2725874/]]] | ||
In the US, cysticercosis is considered by the CDC as one of the five Neglected Parasitic Infections (NPIs). This means that cysticercosis has been targeted as a priority for public health action. | In the US, cysticercosis is considered by the CDC as one of the five Neglected Parasitic Infections (NPIs). This means that cysticercosis has been targeted as a priority for public health action. [[#References|[11]]] From 1990-2002 in the United States, there were 221 cysticercosis deaths. Most of the people who died were Latino, and more were men than women. Nearly 85% of the people who died were foreign born, and 62% had emigrated from Mexico. California accounted for 57% of the total deaths. Only 33 of the 221 deaths occurred in US-born persons. [[#References|[12]]] | ||
Neurocysticercosis is the most common parasitic disease of the nervous system worldwide. It is estimated that more than 1,000 new cases are diagnosed each year in the United States alone. | Neurocysticercosis is the most common parasitic disease of the nervous system worldwide. It is estimated that more than 1,000 new cases are diagnosed each year in the United States alone. [[#References|[9]]] Also, the WHO estimates that of the 50 million people in the world who are affected by epilepsy, 80% of them live in low-income and lower-middle-income countries where <i>T. solium</i> is an endemic [[#References|[13]]]. | ||
==Diagnosis== | ==Diagnosis== | ||
Diagnosis of cysticercosis typically begins when a health care provider asks the patient a series of questions concerning symptoms, location of travel, and possible consumption of contaminated food or water | Diagnosis of cysticercosis typically begins when a health care provider asks the patient a series of questions concerning symptoms, location of travel, and possible consumption of contaminated food or water [[#References|[14]]]. A blood sample will allow for serological testing to be performed, which searches for anticysticercal antibodies in the serum. Currently, enzyme-linked immunotransfer blot (EITB) is the most effective method being used to perform this test. Also, an enzyme-linked immunosorbent assay (ELISA) has been evaluated for the detection of antibodies against <i>T. solium</i> cysticerci in the serum. This method uses the recombinant antigen NC-3 from <i>T. solium</i> cysticerci for the serodiagnosis of cysticercosis, and proves to be a successful form of diagnosis [[#References|[15]]]. Lastly, imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) can be used to detect the presence of cysticercosis, especially neurocysticercosis. These imaging methods might lead to false-positive results, so it is always important to perform a variety of diagnostic tests. [[#References|[15]]] | ||
==Treatment== | ==Treatment== | ||
There a few primary sources of treatment for cysticercosis, and the course of action that is chosen is often influenced by the location of the cysts that have formed. For example, if the cysticercosis is located outside the central nervous system, then surgery can be performed to remove the cysts. [[#References|[16]]] However, if the infection is in the brain (neurocysticercosis), then antiparasitic drugs are normally the preferred treatment. The primary antiparasitc drugs that are used are praziquantel and albendazole. Both of these are effective drugs against <i>T. solium</i> cysticerci. [[#References|[10]]] | |||
Other cases might not require any direct treatment to get rid of the parasite, but intervention is needed to treat the symptoms. These treatments include antiepileptic drugs to treat the seizures often associated with neurocysticercosis and anti-inflammatory drugs to reduce edema associated with the infection. Lastly, some cases of cysticercosis do not require any treatment at all. [[#References|[10]]] | |||
==Prevention== | ==Prevention== | ||
<i>T. solium</i> eggs, which are the causative agent of cysticercosis, are transmitted by the fecal-oral route. Therefore, in order to prevent to spread of these eggs, it is important to partake in proper hand washing techniques after coming in contact with food, water, or other surfaces contaminated with feces. [[#References|[17]]] It is also important to practice good food and water safety especially while travelling in developing countries. This includes drinking bottled water and washing all fruits and vegetables prior to consumption. [[#References|[18]]] | |||
==Host Immune Response== | ==Host Immune Response== | ||
Upon the parasitic invasion, the host mounts an innate and eventually an adaptive immune response. A major component to the host’s defense against the parasite is an inflammatory response. In the case of neurocysticercosis, there are specific celluar types associated with inflammation in the central nervous system (CNS). These include B and T lymphocytes, plasma cells, macrophages, and mast cells. By analyzing the different cell types present, it is clear that the host mounts an innate and adaptive immune response. Depending on the location of the cysticerci, different immunoglobulin isotypes and cytokines will be secreted. For example, if the cysticerci are located in the cerebrospinal fluid (CSF), then there are increased levels of IgG, IgM, IgE, and cytokines IL1β and IL6. Those cytokines both stimulate inflammatory responses. [[#References|[19]]] | |||
However, this inflammatory response can actually harm the host as well. As mentioned earlier, symptoms do not normally occur until the cysticerci are dying. This is due to the host mounting an inflammatory response. Thus, the severe inflammatory host response that occurs during the disruption or death of the parasite is what causes most of the damage created by this infectious disease. [[#References|[5]]] It is predicted that there are a couple cytokines that work to keep inflammation in check. For example, IL10 is believed to regulate the microglial cell production of immune mediators to diminish the pro-inflammatory response induced by the parasite, thus limiting the potentially damaging inflammation within the CNS. [[#References|[19]]] | |||
==References== | ==References== | ||
1 CDC. “Epidemiology & Risk Factors.” <i>Centers for Disease Control and Prevention</i>. 17 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/epi.html.<br> | |||
2 “TAENIA SOLIUM.” <i>Public Health Agency of Canada</i>. 20 April 2012. Web. 21 July 2014. http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/taenia-solium-eng.php.<br> | |||
3 Sorvillo F, Wilkins P, Shafir S, Eberhard M: Public Health Implications of Cysticercosis Acquired in the United States. <i>Emerging Infectious Diseases</i> 2011, 17(1): 1-6.<br> | |||
4 “<i>Taenia</i> Infections.” <i>The Center for Food Security & Public Health</i>. May 2005. Web. 21 July 2014. http://www.cfsph.iastate.edu/Factsheets/pdfs/taenia.pdf.<br> | |||
5 Heyneman D. Cestodes. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 89. Available from: http://www.ncbi.nlm.nih.gov/books/NBK8399/<br> | |||
6 CDC. “Biology.” <i>Centers for Disease Control and Prevention</i>. 22 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/biology.html.<br> | |||
7 White AC Jr, Robinson P, Kuhn R: Taenia solium cysticercosis: host-parasite interactions and the immune response. <i>Chemical Immunology</i> 1997, 66: 209-230.<br> | |||
8 CDC. “Disease.” <i>Centers for Disease Control and Prevention</i>. 14 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/disease.html.<br> | |||
9 Kraft R: Cysticercosis: An Emerging Parasitic Disease. <i>American Family Physician</i> 2007, 76(1):91-96.<br> | |||
10 Garcia HH et al: Current Consensus Guidelines for Treatment of Neurocysticercosis. <i>Clinical Microbiology Reviews</i> 2002, 15(4):747-756. <br> | |||
11 CDC. “Parasites - Cysticercosis.” <i>Centers for Disease Control and Prevention</i>. 16 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/.<br> | |||
12 Sorvillo FJ, DeGiorgio C, Waterman SH: Deaths from Cysticercosis, United States. <i>Emerging Infectious Diseases</i> 2007, 13(2): 230-235.<br> | |||
13 WHO. “Taeniasis/cysticercosis.” <i>World Health Organization</i>. May 2014. Web. 21 July 2014. http://www.who.int/mediacentre/factsheets/fs376/en/.<br> | |||
14 CDC. “Diagnosis.” <i>Centers for Disease Control and Prevention</i>. 14 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/diagnosis.html.<br> | |||
15 Hubert K et al: Serological Diagnosis of Human Cysticercosis by Use of Recombinant Antigens from <i>Taenia solium</i> Cysticerci. <i>Clinical and Diagnostic Laboratory Immunology</i> 1999, 6(4): 479-482.<br> | |||
16 “<i>Taenia solium</i>.” <i>Stanford.edu</i>. Web. 21 July 2014. http://web.stanford.edu/group/parasites/ParaSites2001/taeniasis/solium2.html#management.<br> | |||
17 CDC. “Cysticercosis FAQs.” <i>Centers for Disease Control and Prevention</i>. 17 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/gen_info/faqs.html.<br> | |||
18 CDC. “Prevention and Control.” <i>Centers for Disease Control and Prevention</i>. 24 June 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/prevent.html.<br> | |||
19 Sciutto E et al: The immune response in <i>Taenia solium</i> cysticercosis: protection and injury. <i>Parasite Immunology</i> 2007, 29: 621-636. | |||
Created by | Created by Jordan Voth, student of Tyrrell Conway at the University of Oklahoma. | ||
Latest revision as of 14:35, 11 February 2016

Etiology/Bacteriology
Taxonomy
| Domain = Eukaryota | Phylum = Platyhelminthes | Class = Cestoda | Order = Cyclophyllidea | Family = Taeniidae | Genus = Taenia | species = T. solium
|
NCBI: Taxonomy |
Description
Cysticercosis is an infectious disease that is caused by the larvae of Taenia solium. T. solium is a two-host zoonotic cestode whose adult stage consists of a 2-4 meter long tapeworm. The only known final host for this adult stage is humans, specifically in the small intestine. The intermediate host is the pig, so part of the T. solium life cycle takes place in that host prior to causing taeniasis in humans. Taeniasis is a parasitic infection caused by the presence of tapeworms in the intestine. A person with taeniasis is capable of infecting themselves or others with cysticercosis through fecal-oral transmission. Cysticercosis is transmitted by eating food or drinking water that is contaminated with T. solium eggs. 2-3 months after ingestion, the eggs develop into cysticerci, which are cysts formed by T. solium larvae. The location of the cysts throughout the body determines the symptoms that are demonstrated, diagnoses performed, and treatment used. If the cysts are located in the central nervous system, the infection is specifically called neurocysticercosis.
Cysticercosis is most prevalent in parts of Africa, Asia, Mexico, and Central and South America. However, it is a worldwide issue with about 50 million people having the infection. Because of its fecal-oral transmission, it is important to prevent consumption of contaminated food and water and practice proper hand washing techniques. If the parasite does infect a host, the host immune response will attempt to remove the infection through innate and adaptive responses. Inflammation is a key to the host immune response, yet it can hurt the host as much as it benefits it.
Pathogenesis
Transmission
Larvae of the tapeworm Taenia solium are what cause cysticercosis. If a person becomes contaminated with an adult tapeworm, an infection called taeniasis, they can shed T. solium eggs in their stool. These eggs must then be consumed through fecal-oral route transmission, which can occur by drinking water or eating food that is contaminated with T. solium eggs, or by putting a fecal contaminated object in one’s mouth. Due to this form of transmission, it is possible for autoinfection to occur. If a person has taeniasis, they can infect themselves with the T. solium eggs they shed and thus develop cysticercosis. [1]
Infectious dose, incubation, and colonization
The infectious dose required to cause cysticercosis is relatively unknown [2]. The terminal end of the T. solium tapeworm consists of gravid proglottids, which are full of eggs. Upon the release of these eggs in fecal matter, a person is susceptible to consuming more than one egg. This increases the chances of developing cysticercosis. The median incubation period for cysticercosis is estimated to be about 3.5 years [3] and can range from 10 days to 10 years [4]. A person who has consumed T. solium eggs may go for years without having any symptoms of infection. This is due to the fact that cysticerci often only cause symptoms when they are dying or become large enough to interfere with vital organs. [4] Furthermore, it normally takes 2-3 months for the eggs to develop into cysticerci [2].
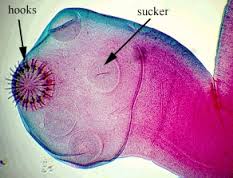
Upon consumption of T. solium eggs, the oncospheres hatch in the intestine and then penetrate the intestinal wall. T. solium contain sharp, spine hooks and four suckers that help them adhere to and penetrate the gut wall [5]. They then migrate to skeletal muscle, the brain, eyes, subcutaneous tissues, and other places where they develop into cysticerci. [6]
Epidemiology
Taenia solium is prevalent in parts of Africa, Asia, Mexico, and Latin America where humans live in close proximity to pigs. This is because pigs are one of the hosts during the life cycle of this two-host parasite, while humans are the other host. When pigs eat T. solium eggs, the eggs develop into larvae and form cysts within the pig’s muscles and other tissues. Then, if humans consume undercooked or raw infected pork, they swallow these cysts. The larvae then come out of their cysts within the human gut and develop into adult tapeworms. Now, this person will shed T. solium eggs in their stool. At this point, if fecal-oral contamination occurs, cysticercosis will develop in whoever consumed the contaminated fecal matter. [1] It is clear that cysticercosis is spread by fecal-oral route, not the consumption of undercooked, infected pork meat.
Virulence factors
T. solium contains a number of virulence factors that allow it to survive amidst the host immune response. The oncospheres invade the host and are susceptible to antibody and complement responses, but by the time the host actually mounts an antibody response, the parasite begins to transform to the more resistant larval stage, called metacestode. During the larval stage, the parasite can evade complement-mediated destruction. This is achieved by a few different virulence factors including paramyosin, which is a protein that inhibits C1q; taeniaestatin, a proteinase, inhibits the classical and alternate pathways; and sulfated polysaccharides, which activate complement away from the parasite. The larval stage of T. solium also manipulates antibody production in such a way that the host produces antibody that cannot kill the mature metacestode, but instead provides a protein source to the parasite. Lastly, taeniaestatin may interfere with lymphocyte proliferation and macrophage functions; therefore, compromising the cellular immune response. [7]
Clinical features
Cysticercosis is an infectious disease that causes the development of cysts, called cysticerci, in various locations of the body [8]. The most popular locations for the cysticerci are the subcutaneous tissues, skeletal muscle, eyes, brain, and spinal cord. The location of the cysts determines a lot about the symptoms, severity, and treatment of the disease. [9]
Symptoms
There are a variety of symptoms that can be demonstrated depending on the location and number of the cysts. If they are located in the skeletal muscle or subcutaneous tissue, there will often times be no signs of symptoms [8]. However, it is sometimes possible to feel lumps and palpable nodules beneath the skin, which may become tender and cause localized pain. If the cysts are located in the eye (usually in the subretinal space or vitreous humor), vision might become blurry and disturbed. This is due to inflammation or retinal detachment. [9] Cysts in the brain or spinal cord cause a specific type of cysticercosis called neurocysticercosis. This is the most severe type of this infectious disease and causes the most threatening symptoms. Symptoms of neurocysticercosis include: headache, seizures, confusion, difficulty with balance, gait disturbances, brain swelling, hydrocephalus (excess fluid around the brain), stroke, and even death [8]. In fact, cysticercosis is the single most common cause of acquired epileptic seizures [10]. Obviously, the symptoms of this disease vary depending on the location of the cysts.
Morbidity and Mortality
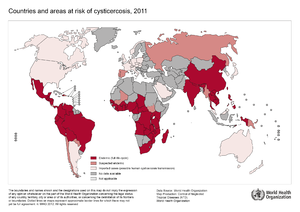
Cysticercosis is the most common parasitic disease across the world, and it is estimated that 50 million people are infected with it. The highest rates of infection for cysticercosis are found in parts of Africa, Asia, Mexico, and Central and South America. [9] However, it is becoming more of an issue in the United States because of increased immigration and world travelling. The listed locations tend to have poor sanitation and free-roaming pigs, which are environments that Taenia solium thrive and fecal-oral contamination can more easily occur. [11]
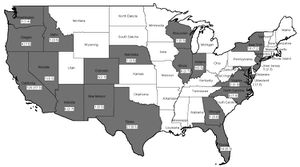
In the US, cysticercosis is considered by the CDC as one of the five Neglected Parasitic Infections (NPIs). This means that cysticercosis has been targeted as a priority for public health action. [11] From 1990-2002 in the United States, there were 221 cysticercosis deaths. Most of the people who died were Latino, and more were men than women. Nearly 85% of the people who died were foreign born, and 62% had emigrated from Mexico. California accounted for 57% of the total deaths. Only 33 of the 221 deaths occurred in US-born persons. [12]
Neurocysticercosis is the most common parasitic disease of the nervous system worldwide. It is estimated that more than 1,000 new cases are diagnosed each year in the United States alone. [9] Also, the WHO estimates that of the 50 million people in the world who are affected by epilepsy, 80% of them live in low-income and lower-middle-income countries where T. solium is an endemic [13].
Diagnosis
Diagnosis of cysticercosis typically begins when a health care provider asks the patient a series of questions concerning symptoms, location of travel, and possible consumption of contaminated food or water [14]. A blood sample will allow for serological testing to be performed, which searches for anticysticercal antibodies in the serum. Currently, enzyme-linked immunotransfer blot (EITB) is the most effective method being used to perform this test. Also, an enzyme-linked immunosorbent assay (ELISA) has been evaluated for the detection of antibodies against T. solium cysticerci in the serum. This method uses the recombinant antigen NC-3 from T. solium cysticerci for the serodiagnosis of cysticercosis, and proves to be a successful form of diagnosis [15]. Lastly, imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) can be used to detect the presence of cysticercosis, especially neurocysticercosis. These imaging methods might lead to false-positive results, so it is always important to perform a variety of diagnostic tests. [15]
Treatment
There a few primary sources of treatment for cysticercosis, and the course of action that is chosen is often influenced by the location of the cysts that have formed. For example, if the cysticercosis is located outside the central nervous system, then surgery can be performed to remove the cysts. [16] However, if the infection is in the brain (neurocysticercosis), then antiparasitic drugs are normally the preferred treatment. The primary antiparasitc drugs that are used are praziquantel and albendazole. Both of these are effective drugs against T. solium cysticerci. [10]
Other cases might not require any direct treatment to get rid of the parasite, but intervention is needed to treat the symptoms. These treatments include antiepileptic drugs to treat the seizures often associated with neurocysticercosis and anti-inflammatory drugs to reduce edema associated with the infection. Lastly, some cases of cysticercosis do not require any treatment at all. [10]
Prevention
T. solium eggs, which are the causative agent of cysticercosis, are transmitted by the fecal-oral route. Therefore, in order to prevent to spread of these eggs, it is important to partake in proper hand washing techniques after coming in contact with food, water, or other surfaces contaminated with feces. [17] It is also important to practice good food and water safety especially while travelling in developing countries. This includes drinking bottled water and washing all fruits and vegetables prior to consumption. [18]
Host Immune Response
Upon the parasitic invasion, the host mounts an innate and eventually an adaptive immune response. A major component to the host’s defense against the parasite is an inflammatory response. In the case of neurocysticercosis, there are specific celluar types associated with inflammation in the central nervous system (CNS). These include B and T lymphocytes, plasma cells, macrophages, and mast cells. By analyzing the different cell types present, it is clear that the host mounts an innate and adaptive immune response. Depending on the location of the cysticerci, different immunoglobulin isotypes and cytokines will be secreted. For example, if the cysticerci are located in the cerebrospinal fluid (CSF), then there are increased levels of IgG, IgM, IgE, and cytokines IL1β and IL6. Those cytokines both stimulate inflammatory responses. [19]
However, this inflammatory response can actually harm the host as well. As mentioned earlier, symptoms do not normally occur until the cysticerci are dying. This is due to the host mounting an inflammatory response. Thus, the severe inflammatory host response that occurs during the disruption or death of the parasite is what causes most of the damage created by this infectious disease. [5] It is predicted that there are a couple cytokines that work to keep inflammation in check. For example, IL10 is believed to regulate the microglial cell production of immune mediators to diminish the pro-inflammatory response induced by the parasite, thus limiting the potentially damaging inflammation within the CNS. [19]
References
1 CDC. “Epidemiology & Risk Factors.” Centers for Disease Control and Prevention. 17 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/epi.html.
2 “TAENIA SOLIUM.” Public Health Agency of Canada. 20 April 2012. Web. 21 July 2014. http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/taenia-solium-eng.php.
3 Sorvillo F, Wilkins P, Shafir S, Eberhard M: Public Health Implications of Cysticercosis Acquired in the United States. Emerging Infectious Diseases 2011, 17(1): 1-6.
4 “Taenia Infections.” The Center for Food Security & Public Health. May 2005. Web. 21 July 2014. http://www.cfsph.iastate.edu/Factsheets/pdfs/taenia.pdf.
5 Heyneman D. Cestodes. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 89. Available from: http://www.ncbi.nlm.nih.gov/books/NBK8399/
6 CDC. “Biology.” Centers for Disease Control and Prevention. 22 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/biology.html.
7 White AC Jr, Robinson P, Kuhn R: Taenia solium cysticercosis: host-parasite interactions and the immune response. Chemical Immunology 1997, 66: 209-230.
8 CDC. “Disease.” Centers for Disease Control and Prevention. 14 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/disease.html.
9 Kraft R: Cysticercosis: An Emerging Parasitic Disease. American Family Physician 2007, 76(1):91-96.
10 Garcia HH et al: Current Consensus Guidelines for Treatment of Neurocysticercosis. Clinical Microbiology Reviews 2002, 15(4):747-756.
11 CDC. “Parasites - Cysticercosis.” Centers for Disease Control and Prevention. 16 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/.
12 Sorvillo FJ, DeGiorgio C, Waterman SH: Deaths from Cysticercosis, United States. Emerging Infectious Diseases 2007, 13(2): 230-235.
13 WHO. “Taeniasis/cysticercosis.” World Health Organization. May 2014. Web. 21 July 2014. http://www.who.int/mediacentre/factsheets/fs376/en/.
14 CDC. “Diagnosis.” Centers for Disease Control and Prevention. 14 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/diagnosis.html.
15 Hubert K et al: Serological Diagnosis of Human Cysticercosis by Use of Recombinant Antigens from Taenia solium Cysticerci. Clinical and Diagnostic Laboratory Immunology 1999, 6(4): 479-482.
16 “Taenia solium.” Stanford.edu. Web. 21 July 2014. http://web.stanford.edu/group/parasites/ParaSites2001/taeniasis/solium2.html#management.
17 CDC. “Cysticercosis FAQs.” Centers for Disease Control and Prevention. 17 April 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/gen_info/faqs.html.
18 CDC. “Prevention and Control.” Centers for Disease Control and Prevention. 24 June 2014. Web. 21 July 2014. http://www.cdc.gov/parasites/cysticercosis/prevent.html.
19 Sciutto E et al: The immune response in Taenia solium cysticercosis: protection and injury. Parasite Immunology 2007, 29: 621-636.
Created by Jordan Voth, student of Tyrrell Conway at the University of Oklahoma.
