Deep sea vent
Template:Biorealm Niche: Deep Sea Vent
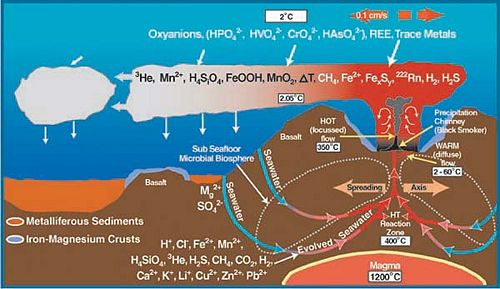
Description of Niche
Hydrothermal vents, also known as deepwater seeps, deep-sea springs, and deep sea vents are the aftermath of a volcanic eruption due to shifting of the plates that form the Earth’s crust . The shifting causes cracks to form when the earth’s plates are pulled apart along the Mid-Ocean Ridges[[6]]. This allows water to seep directly into the cracks and become heated by the magma chambers up to around 400oC. Typical to these sites are the columnar chimneys, black smokers, and white smokers that are formed due to the high pressure at this depth and the temperature of the trapped water. The hot water is forced out of the cracks dissolving minerals and chemicals from the rocks, which forms a chemical plume (Jones, M.).

Within a year, what flow through these now mature sulfide chimneys are high-temperature fluids. The chimneys form to a height of 10 to 20 meters. The black smokers emit fluids at 400oC or above causing it to emit chemicals such as sulfide, iron, copper and zinc. When the chemicals and the hot water interact with the low pH of the surrounding water, black precipitation occurs, giving black smokers its name. The white smokers emit fluids at 100-300oC in temperature. At the lower temperatures, the silica, anhydrite, and barite precipitate as white particles instead of black (Van Dover, C.).
Hydrothermal-vent fields range from several hundred to several million square meters. It is also because of the larger range of the vent fields that allows for the low-temperature diffuse flows. It is the warm-water diffuse that allows for the sustainability of productive populations and organisms.
Although some hydrothermal vent organisms have adapted to the high temperatures, it is the chemistry of the fluids, which takes place because of the high temperature, that sustains the chemosynthetic basis of life at hydrothermal vent ecosystems (Van Dover, C.).
Where located?
Many of these hydrothermal vents are found along the Juan de Fuca, East Pacific Rise, Gorda, Galapagos, Hawaiian and Explorer Ridges, Mid-Atlantic Ridge, Mariana Trough, Okinawa Trough, Izu-Ogazawara Arc, and Central and Southeast Indian Ridges (Desbruyeres, D., Segonzac, M., and Bright, M.)[[7]].
The depth of the locations of these hydrothermal vents varies from 2000 meters found on the Galapagos Ridge, to 7,700 meters found on the Mid-Atlantic Ridge [[8]].
Influence by Adjacent Communities
Due to the spread of smoker activity, neighboring deep sea communities may be affected by the high temperature and chemical toxicity of its fluid. If the neighboring communities have not adapted to the high temperatures there is a very likely chance they won’t survive. Examples of dead clam communities have been found at various sites close by these hydrothermal vents (Ernst et al., 1982). Studies have also shown that once hydrothermal vent activity occurs, many predators tend to die due to toxicity and temperature changes. Since most predators feed off of small gastropods and sessile invertebrates including vestimentiferan worms and mussels, the loss of these predators causes an increased presence of benthic invertebrates, mobile gastropods, and amphipod crustaceans that would normally survive in smaller communities (Micheli et al., 2002).
Conditions under which the environment changes
Depending on its activity, smoker vents can increase in size and spread from 5-9 cm per year or as fast as 9-16 cm per year (Jones, M.). The activity can be affected by the rock composition, sediments available, permeability of the ocean crust, amount of time between separation of vapor and liquid after exposure to high temperatures, time elapsed since last activity, and how deep the heat source which can cause a shift in its environment. Also, hydrothermal deposits are found in increasing amounts towards the north as a result of increased tectonic deformation of the ridges due to the decreasing distance from its neighboring continents (Butterfield, D.).
Physical Conditions?
Who lives there?
Microbes Present
Within the hydrothermal vents of the deep sea, a myriad of bacteria and archaea live and prosper, despite being surrounded by heat, pressure and lack of light (Botos). These bacteria respond by using certain processes, described later, which enable them to survive.
There may be aproximately ten to twenty thousand species of bacteria and archaea that roam the deep sea vents (Botos). Most of them rely on chemosynthesis, a process by which fairly useless chemical compounds are transferred into new compounds with the ability to be used as energy for other organisms. This process is very similar to the ability of plants to turn compounds into energy, photosynthesis (Wirsen et. al.).
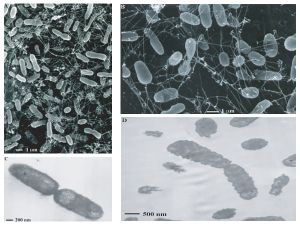
Studies have classified entire archaeal communities in deep-sea hydrothermal vent chimney structures (Takai K, Komatsu T, Inagaki F, and Horikoshi K). Scientists discovered deep-sea hydrothermal vents in 1979.
The majority of the microbes that live in this niche include hyperthermophiles and thermophiles from both the bacterial and archaeal domains. Recent studies have shown an increasing number of unclassified and uncultivated thermophiles. This leads scientists to believe that these communities are very phylogenetically diverse. Major types of bacteria that live near these vents are mesophilic sulfur bacteria. These bacteria are able to achieve high biomass densities due to their unique physiological adaptations. For example, Beggiatoa spp. is able to carry an internal store of nitrate as an electron acceptor that helps with the harvesting of free sulfide in the upper sediment region of the vents.
Recent studies have shown that large populations of extremely halophilic archaea inhabit the inside structures of black smoker chimneys. These bacteria belong to the genera Halomonas and Marinobacter. The existence of these halophilic archaea is probably due to the brines/salt deposits found in deep-sea hydrothermal systems (Takai K, Komatsu T, Inagaki F, and Horikoshi K).
Based on microbiological, geochemical, and geophysical observations, some scientists believe that a whole new biosphere exists beneath active hydrothermal vents. This idea is supported by the detection of microbial rDNA in the black smoker vent water. However, it is difficult to conclude if there is a true microbial population living under black smoker vents because deep ocean water is continuously being filtered underneath sea floor basalts and pumped out of black smoker vents (Takai K, Komatsu T, Inagaki F, and Horikoshi K).
Some more of the bacteria and archaea found in the hydrothermal deep sea vents are:
Methanocaldococcus jannaschii (previously from the genus Methanococcus): Methanocaldococcus jannaschii, a hyperthermophilic, hydrogenotrophic, and methanogenic archaea (meaning it produces methane [methanogenesis]), is one of the many microbes inhabiting the hydrothermal vents. The cold seawater surrounding the deep sea vents, permeates through the chimney and lowers the temperature from 350°C, to a temperature M. jannaschii can survive. By permeating through the chimney wall, oxygen is also brought into the nutrient rich vent fluid. M. jannaschii uses sulfide (S2-) for growth and energy, which is good because sulfide is present in high levels in the vent fluid. It reacts with oxygen to establish anaerobic growth conditions for the archaea. However, sometimes sulfite (SO32-) is potentially produced. This is problematic because sulfite is toxic and inactivates methyl-coenzyme M reductase, which is essential to methanogenesis. When sulfite is present, M. jannaschii produces the coenzyme F420-dependent sulfite reductase (Fsr), which reduces sulfite to sulfide. Fsr both protects the cell from sulfite and gives it an anabolic ability. (Johnson).
Desulfonauticus submarinus: one of the most novel organisms at the deep sea vent and one of the newest to be found. It has been given precedence in terms of its possible significance in ecological terms, mainly because of its symbiosis with the deep sea worms Alvinella and Riftia. This bacterium also uses sulfur-containing compounds to change them into energy. In this case, the bacterium is a sulfur-reducer, meaning it decreases the oxidation state of the element of sulfur within each compound. Using mostly sulfates, it transforms these compounds into sulfites, sulfides, thiosulfates and even elemental sulfur (S8), all which have lower oxidation states, and therefore higher numbers of electrons, to be used by organisms such as Alvinella, the Pompeii worm, or Riftia, the giant tube worm, on the ocean floor (Audiffrin et. al.).
Candidatus Arcobacter sulfidicus: one of the group of bacteria considered to be under the heading Proteobacteria. Candidatus has been found in other places and is considered a great bacterium for the production of sulfur using an oxidative process. It takes up sulfides and then oxidizes them (increasing oxidation state) into filamentous sulfur as the end product. This is used also as an energy source and is interesting due to its’ possible link to the use of the Calvin cycle, known from the photosynthetic pathways of the plant kingdom, but this has yet to be proven (Wirsen et. al.). It also has an above average capability of going through CO2 fixation, a beneficial process for other organisms within the habitat.
Desulfovibrio vulgaris, Desulfovibrio gigas, Desulfobacterium autotrophicum, and Desulfobacter latus: These four bacteria are categorized within the epsilon (ε) class of the Proteobacteria. These organisms actually live on the outside of another member of the animal kingdom, Alvinella pompejana, or the Pompeii worm. On the dorsal integument, or the exterior, live these bacteria, doing the same job as their counterparts from the vents themselves by reducing and oxidizing sulfur-containing compounds. The most important discovery, though, was that the bisulfite reductase genes of these bacteria were different from each other, leading to believe that oxygen is not necessary and therefore, anaerobic sulfate-reducing bacteria can thrive. Dissimilatory bisulfite reductase is the terminal redox enzyme that catalyzes the reduction of sulfite to sulfide during anaerobic respiratory sulfate reduction. As to this theory, there has been no conclusive evidence but scientists are still working on an answer. They also help to detoxify the worm’s system of possibly metals and hydrogen sulfide, though this is not entirely clear as of yet (Cottrell et. al.).
Non-microbes present
In addition to the thousands of different microbe organisms freely flowing throughout the deep sea vents, there are many other organisms, mostly from the animal kingdom, which make the vents their habitat (Botos). The attraction from other organisms comes from the thick gathering of bacteria at the opening of what is called the “black smoker” vent (a type of hydrothermal vent, resembling a black chimney-like structure), where the bacteria gain the most advantages. Here, the microbes can gather their sulfur containing material and can exude the energy necessary for organisms to survive in the very harsh thermophilic and hyperbaric environment (Tivey).
Some of the organisms that live at the hydrothermal deep sea vents are:
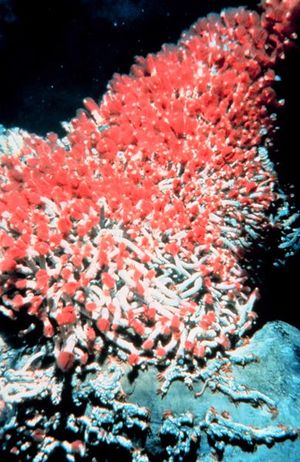
Tevnia jerichonana and Riftia pachyptila: these are the two types of tube worms that inhabit the deep sea vents and thrive in such an unlikely environment. These tube worms do not have a true digestive tract or even a mouth; instead the bacteria inhabit the interior of the entire body and live there. These worms have red plumes (the color comes from the worm’s hemoglobin), which bring in nutrients for the symbiotic bacteria to thrive on (the bacteria are inside the worm on a structure called a trophosome). Taking these nutrients in, the bacteria can then start processing the organic molecules the worm needs for energy. This symbiotic relationship is seen throughout the deep sea vent system (“Extremes of Eel City”).
Alvinella pompejana: this is known as the Pompeii worm; it can withstand enormous amounts of heat and therefore thrives in this inhospitable environment. It resides just outside of the opening of the vent and uses its’ tube to its’ advantage; one side (the head) stays in the cooler water to take up food and nutrients, while the other side (the tail) stays in the extreme heat. This is one of the most studied thermophiles of the deep sea and is one of the best examples of symbiosis at the vents. Instead of an absent digestive tract, Alvinella uses its’ red plume on its’ dorsal side to release the hemoglobin, and in turn, the sulfur containing compounds. The bacteria use the plume as a home base to turn this into compounds yielding energy to the worm. Studies have shown a possible heat-absorbing factor of the symbiotic bacteria on the back of the worm; this can be why Alvinella can withstand such an enormous amount of heat (Cottrell et. al.).
Other existing organisms that have been found in vent communities (Desbruyeres et al., 2006):

There are many other organisms, such as snails, shrimp, crabs, fish, and octopi which form a food chain of predator and prey relationships. These marine organisms do not specifically use the bacteria, but through the food chain, may benefit from the bacteria and its energy yielding capability through chemosynthesis (Goffredi et. al.).
Microbe interaction with each other or non-microbes
The hydrothermal vent tubeworm Riftia pachyptila is well-known for its symbiotic relationship with sulfide oxidizing chemoautotrophic bacteria found in the cells of its trophosome tissue. The hemoglobin in the worms combines hydrogen sulfide and then gives this product to the bacteria. The bacteria, in return, give back carbon compounds to the worm. This interaction requires specific communications mechanism in both the bacteria and the worms. Scientists have found two classes of genes from Riftia symbionts that encode for environmental sensors, response regulators, and components of bacterial chemotaxis systems.
In detail, scientists have found functional genes encoding the following: members of the two-competent regulatory family, the methyl-accepting chemotaxis protein, and the flagellar C protein of the eubacterial flagellum. These functional genes strongly support the idea that these bacteria have a motile, free stage and are then acquired by Riftia each new generation (Hughes D, Felbeck H, Stein J).
As far as the evidence shows, all the microbes involved within the deep sea vent ecosystem help the environment to survive and to thrive without the use of light, a key factor for other organisms in the ocean. Also, extremes such as heat, pressure and less nutrients play a role in how the microbes can adapt to the vents almost five to ten thousand feet below sea level. As there are so many types of bacteria within the ecosystem, of course there will be plenty of interaction, but as to what type of interaction is not quite clear (Matz). It is very difficult to examine these bacteria up close and bringing them (including the symbiotic organisms that depend on these bacteria) out of their habitat may perhaps permanently damage crucial bacterial pathways, and may not survive the decompression of the ocean’s high pressure (Matz).
That being said, having similar bacteria within one organism perhaps shows some type of interaction, but it is unclear at this time as to what that could be. As stated above, the Desulfo bacteria (Desulfovibrio vulgaris, Desulfovibrio gigas, Desulfobacterium autotrophicum, and Desulfobacter latus) are all contained within the intestine of the annelid Alvinella pompejana but as to what they may have in common with each other, it is still unclear (Cottrell et. al.). One thing that is known presently is that there is no evidence of negative behavior where one microbe competes with another for an advantage or at least, nothing that can be found in the literature. They all add their individual parts to make the whole system work as one.
Microbes changing their environment
Through the process of chemosynthesis, the bacteria and archaea transform sulfur containing materials (by either reducing or oxidizing the compound) into other chemical mixtures that other deep sea organisms can use (Cottrell et. al., Johnson et. al., Goffredi et. al.). Some bacteria may also create that primary sulfur, so that it can continue the process along within the hydrothermal vents, but mostly this sulfur supply comes from organisms themselves, who use hemoglobin as a way to achieve symbiosis with the microbes (Cottrell et. al.).
Through these processes, the pH of the substances the microbes work on changes, fluctuating from very acidic to very basic, and vice versa, as the compound can be reduced or oxidized (Audriffin et. al., Johnson et. al., Wirsen et. al.). Some of these microbes inhabit the insides of their symbiotic organisms, within the intestinal tract or esophageal cavity, or even when a digestive tract is absent, for example. Also, sulfur and methane are not the only substances that are released by the bacteria; certain minerals, such as iron, calcium and phosphorus can also be released and used by other organisms within their metabolism (Cottrell et. al., Goffredi et. al., Jannasch et. al.).
Microbe metabolism affecting the environment
The primary source of metabolism for providing food is through animal-bacteria symbiosis. These bacteria are typically chemolithotrophic bacteria. In many worms, they have a layer of tissue called trophosome that fills the body cavity and allows these chemolithotrophic bacteria to live symbiotically in these trophosomes where they can oxidize sulfide. Enzymes in the trophosome also have the capacity to oxidize hydrogen sulfide. The energy produced can be used to drive net fixation of CO2 and to reduce nitrate to ammonia. A mechanism to avoid poisoning aerobic respiration by hydrogen sulfide is protected by sulfide binding proteins in the blood. The idea is to prevent as little free floating sulfide as possible (SV et al., 2008).
Many invertebrates also show a range of O2 consumption that is similar to species that live closer to the surface. Besides a difference in thermal effects, there is no decline of O2 consumption, strongly indicating the importance of endosymbionts. Other organisms, such as deep sea pelagic animals will show a lower O2 consumption due to its inability to swim. If they lose the ability to swim, they can save that energy and lower their O2 consumption (Jones, M.).
The microbes involved in the hydrothermal vents are sulfur-reducers as well as sulfur-oxidizers. This means that the sulfur element (the “S” in all of the compounds) either goes down in oxidation state (more negative i.e. more electrons gained) or goes up in oxidation state (less negative i.e. more electrons lost). Through this catabolism and anabolism of sulfur compounds, energy is also produced, which is transferred, accordingly, to the more advanced organisms which do not produce their own energy. This process is the most necessary in order for the deep sea vent system to thrive. The bacteria present here take the place of plants near the surface of the ocean or even on land. As photosynthesis converts light from the sun into energy for the animals (for example, oxygen and glucose), chemosynthesis is at full power deep in the sea where light cannot reach. These microbes as well as the organisms around them depend on the compounds made, either by the organisms themselves (for example, Alvinella), or by the surrounding vents, in order to become more energy-efficient. (Cottrell et. al., Goffredi et. al., Jannasch et. al., Wirsen et. al.).
The nitrogen cycle transforms nitrogen and nitrogen-containing compounds in nature. Nitrification is a step of the nitrogen cycle that oxidizes ammonium into nitrates. The anaerobic oxidation of ammonium (NH4) is known as anammox. Anammox bacteria have been observed in a large range of temperature habitats. Studies have shown that anammox have also played a major role in the hydrothermal settings of deep-sea vents. A recent study used anammox-specific primers and 16S rRNA gene sequences were retrieved from different samples of hydrothermal vents. Hydrothermal vent chimneys are typically hot and anaerobic environments and this is generally a place where suitable amounts of nitrites and ammonium are present. This study proves diversity of prokaryotes and a basic understanding of the metabolism of the prokaryotes in deep-sea vents (Byrne et. al.).
Current Research
1. Current research is being done to learn more about photosynthesis evolution. Some scientists believe that photosynthesis evolved from geothermal vents and then sunlight. This idea raises the possibility that photosynthesis originated from deep-ocean hydrothermal vents and then dispersed upwards to shallow-waters and more sunlight. The first photosynthetic bacteria to be found living at deep sea vents were discovered in 1998. The overall question was: what were the morphological and physiological properties of this new bacterium.
They addressed this question by extracting samples from non-buoyant regions of plumes emitted from the hydrothermal vents. The scientists then brought the samples on board the ship and stored them in sterile bottles at 4 degrees Celsius. The scientists plated the bacteria and incubated them aerobically at room temperature in the dark for 5 days. After the colonies were identified, the bacteria were incubated at 30 degrees Celsius for another 5 days. The bacteria’s capability for anaerobic photosynthetic growth was tested in screw-cap test tubes and in agar by using media for purple sulfur and non-sulfur bacteria. However, none of the strains tested were able to grow anaerobically in light.
This particular species had bacterial chlorophyll alpha and carotenoids. It is a member of the aerobic anoxygenic photosynthetic bacteria due to its following characteristics: the inability to grow anaerobically in the light, the small number of photosynthetic units it has, and its abundance of carotenoids. Aerobic anoxygenic photosynthetic bacteria belong under the subclass of Proteobacteria.
Aerobic anoxygenic photosynthetic bacteria are different in comparison to purple sulfur or non-sulfur bacteria, because they can utilize light as a source of energy for anaerobic growth. Photosynthetic activity in these bacteria is shown by the following three characteristics: the reversible photo-oxidation of cytochromes and the reversible photo-bleaching of bacterial chlorophyll, photo-inhibition of respiration, and light-stimulated increase in ATP pools which lead to an increase in growth rate and biomass production. The scientists concluded that light is a secondary source of energy for these bacteria.
2. α-Amylase is an enzyme that hydrolyzes starch by cleaving α-1,4-glucosidic linkages at random sites. This enzyme is widely used in starch-processing, brewing, alcohol production, textile, and other different industries. α-Amylase is one of the most important commercial enzymes. The most thermostable α-amylase that is used in industries is produced from a microbe called Bacillus licheniformis. The enzyme produced by this microbe operates optimally at 90°C and at a pH of 6.0, but it also requires addition of calcium ion, Ca2+, for maintaining its thermostability.
Starch bioprocessing has two steps, liquefaction and saccharification. The most ideal conditions for these two steps are for them to be performed at 105°C and pH 4.5. However, the α-Amylase from Bacillus licheniformis is the best enzyme to use for the liquefaction step and the pH must be raised to 5.7-6.0 and calcium salt needs to be added for thermostability. Then the saccharification step uses a glucoamylase isolated from an Aspergillus sp. which requires the pH to return to 4.5 to work efficiently. These two modification steps increase chemical costs and create additional refining steps to remove the calcium salt from the final product. If an α-amylase that is able to work at pH 4.5 and 105°C without the addition of calcium is to be found then it would greatly reduce costs and simplify the process of starch bioprocessing.
An extremely thermophilic anaerobic archaeon strain of Thermococcus, HJ21, was isolated from a deep-sea hydrothermal vent. This strain was found to be able to produce hyperthermophilic α-amylase named THJA (Thermococcus HJ21 amylase). The HJ21 strain was isolated and grown on different mediums to determine the optimal growth conditions. The genomic DNA was extracted and the 16S rRNA was amplified and sequenced. Protein purification of the α-amylase was performed to test the effects of pH and temperature on the activity and stability of THJA. The extracellular thermostable THJA is found to be most efficient at pH 5.0 and at a temperature of 95°C. This enzyme also did not require calcium ion for thermostability. This newly found enzyme opens the door of opportunity to find a much more efficient way to process starch. It can help reduce the cost and greatly reduce the amount of work needed in the bioprocessing of starch. This can lead to development for better efficiency in other industries that use α-amylase (Wang, S., Lu, Z., Lu, M., Qin, S., Liu, H., Deng, X., Lin, Q., and Chen, J).
3. DNA bacteriophages lyse the host cell by using a two component lytic system consisting of two proteins, holin and lysin. Holins are small proteins without any known enzymatic function other than controlling the timing of lysis and to form pore in the cytoplasmic membrane of the host. These pores allow lysin to access the peptidoglycan layer of the host cell. Lysins are proteins with one of several muralytic activities, responsible for the destruction of the peptidoglycan.
The destruction of peptidoglycan plays an important role in the infection of the bacteriophage. When the bacteriophage infects a host bacterium, the lysin of the bacteriophage digests the cell wall of the host from the outside. Then, the phage injects its genome into the host bacterial cell. A lot of lysins that have been found are obtained from bacteriophages that infect mesophiles. However, very few lysins are obtained from thermophilic bacteriophage. The thermophilic bacteriophages from deep-sea hydrothermal vents will greatly improve the study and understanding of the lysis mechanism of bacteriphages under thermophilic conditions.
In a recent study, a lysin-encoding gene was cloned from a deep-sea thermophilic bacteriophage Geobacillus virus E2, GVE2. After recombination and expression of the lysin gene in E. coli the GVE2 lysin was purified. The lysin was highly active when enzyme inhibitors or detergents were present but was strongly inhibited by sodium dodecyl sulfate and ethylene di-amine tetra-acetic acid. The lysin’s enzymatic activity was also slightly stimulated by Na+ and Li+ but slightly inhibited by metal ions like Mg2+, Ba2+, Zn2+, Fe3+, Ca2+, and Mn2+. This study was the first time the characterization of lysin was obtained from deep-sea thermophilic bacteriophage. This can open up bacteriophage lysins as possible antimicrobial agents against gram-positive bacteria. There is also potential for the use of lysins as a prophylaxis and treatment of bacterial pathogens. Since peptidoglycan is not found in eukaryotic cells, lysins can be expected to be well tolerated by humans. This opens up opportunities for research to use lysin as antimicrobial agents in protecting plants and crops and even in food (Ye, Ting and Zhang, Xiaobo).
Summary
Before, life was considered nonexistent around deep sea vents. However, exploration of the deep sea vents revealed thriving organisms that have adapted to high pressure, rapidly changing temperatures, and high toxicity. The primary adaptation for these organism is through symbiosis. Many of these sulfide reducing microbes live within invertebrates. These microbes convert sulfide into a usable source of energy. Current research is ongoing to better understand how these organisms survive and interact in such a harsh environment.
References
Audiffrin, Carine, Cayol, Jean-Luc, Joulian, Catherine, Casalot, Laurence, Thomas, Pierre, Garcia, Jean-Louis, and Ollivier, Bernard. “Desulfonauticus submarines gen. nov., sp. Nov., a novel sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent”. International Journal of Systematic and Evolutionary Microbiology. 2003. Volume 53. pp. 1585-1590. Botos, Sonia. "Life on a hydrothermal vent”. © 2005. <http://www.botos.com/marine/vents01.html#body_4>. Butterfield, David A. "Submarine Ring of Fire: Background." Ocean Explorer/Explorations. 11 July 2005. NOAA and US Department of Commerce. 27 Aug. 2008 <http://oceanexplorer.noaa.gov/explorations/02fire/background/vent_chem/ventchem.html>.Byrne, N., Strous, M., Crepeau, V., Kartal, B., Birrien, J., Schmid, M., Lesongeur, F., Schouten, S., Jaeschke, A., Jetten, M., Prieur, D., and Godfroy, A. “Presence and activity of anaerobic ammonium-oxidizing bacteria at deep-sea hydrothermal vents”. International Society for Microbial Ecology. 31 July 2008.
Cottrell, Matthew T., and Cary, S. Craig. “Diversity of Dissimilatory Bisulfite Reductase Genes of Bacteria Associated with the Deep-Sea Hydrothermal Vent Polychaete Annelid Alvinella pompejana”. Applied and Environmental Microbiology. 1999. Volume 65. pp. 1127-1132. Desbruyeres, Daniel, Michael Segonzac, and Monika Bright. Handbook of Deep-Sea Hydrothermal Vent Fauna. Linz: Medieninhaber und Herausgeber, 2006. Ernst, W. G., and J. G. Morin. The Environment of the Deep Sea. Vol. II. Englewood Cliffs, NJ: Prentice-Hall Inc., 1982. “Extremes of Eel City”. Astrobiology Magazine. 2005. <http://www.astrobio.net/news/article1577.html>. Goffredi, Shana K., Waren, Anders, Orphan, Victoria J., Van Dover, Cindy L., and Vrijenhoek, Robert C. “Novel Forms of Structural Integration between Microbes and Hydrothermal Vent Gastropod from the Indian Ocean”. Applied and Environmental Microbiology. 2004. Volume 70. pp. 3082-3090. Hughes D, Felbeck H, Stein J. Signal Transduction Pathways in the Endosymbiont of the Hydrothermal Vent Tubeworm Riftia pachyptila. Talk Jannasch, Holger W., Wirsen, Carl O., Molyneaux, Stephen J., and Langworthy, Thomas A. “Extremely Thermophilic Fermentative Archaebacteria of the Genus Desulfurococcus from Deep-Sea Hydrothermal Vent”. Applied and Environmental Microbiology. 1988. Volume 54. pp. 1203-1209. Johnson, Eric F., and Mukhopadhyay, Biswarup. “A New Type of Sulfite Reductase, a Novel Coenzyme F420-dependent Enzyme, from the Methanarchaeon Methanocaldococcus jannaschii”. The Journal of Biological Chemistry. 2005. Volume 280. pp. 38776-38786. Jones, Meredith L. Hydrothermal Vents of the Eastern Pacific: An Overview. 6th ed. Vienna, VA: INFAX Corporation, 1985. Matz, Mike. “The Worm that Boasts Earth’s Hottest Lifestyle”. Exploratorium Dispatches. 2001. <http://www.exploratorium.edu/aaas-2001/dispatches/thermal_worm.html> Micheli, Fiorenza, Charles H. Peterson, Lauren S. Mullineaux, Charles R. Fisher, Susan W. Mills, Gorka Sancho, Galen A. Johnson, and Hunter S. Lenihan. "Predation Structures Communities At Deep-Sea Hydrothermal Vents." Ecological Monographs 72 (2002): 365-82. Nakagawa, Satoshi, Takaki, Yoshihiro, Shimamura, Shigeru, Reysenbach, Anna-Louise, Takai, Ken, and Horikoshi, Koki. "Deep-sea vent ε-proteobacterial genomes provide insights into emergence of pathogens". Proceedings of the National Academy of Sciences of the United States of America. 2007. Volume 104(29). pp. 12146-12150. Nelson, Douglas C. Recent Findings in the Microbiology of Hydrothermal Vents and Seeps. Invited Talk Scearce, Carolyn. "Hydrothermal Vent Communities." Discovery Guides. May 2006. CSA. 25 Aug. 2008 <http://www.csa.com/discoveryguides/vent/review.pdf>. SV, Nyholm, Robidart J., and Girquis PR. "Coupling metabolite flux to transcriptomics: insights into the molecular mechanisms underlying primary productivity by the hydrothermal vent tubeworm Ridgeia piscesae." Biological Bulletin (2008): 255-65. <http://www.ncbi.nlm.nih.gov/pubmed/18574102?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum> Takai K, Komatsu T, Inagaki F, and Horikoshi K. <http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=11472939>. Appl Environ Microbiol. 2001 August; 67(8): 3618–3629 Tivey, Margaret K. “How to Build a Black Smoker Chimney: The Formation of Mineral Deposits At Mid-Ocean Ridges”. Woods Hole Oceanographic Institution. 1998. <http://www.whoi.edu/oceanus/viewArticle.do?id=2400>. Van Dover, Cindy Lee. The Ecology of Deep-Sea Hydrothermal Vents. Princeton: Princeton UP, 2000. Wang, S., Lu, Z., Lu, M., Qin, S., Liu, H., Deng, X., Lin, Q., and Chen, J. “Identification of archaeon-producing hyperthermophilic α-amylase and characterization of the α-amylase”. Applied Microbiology and Biotechnology. 28 Jun 2008. Wirsen, C.O., Sievert, S.M., Cavanaugh, C.M., Molyneaux, S.J., Taylor, L.T., DeLong, E.F., and Taylor, C.D. “Characterization of an Autotrophic Sulfide-Oxidizing Marine Arcobacter sp. That Produces Filamentous Sulfur”. Applied and Environmental Microbiology. 2002. Volume 68. pp. 316-325. Ye, Ting and Zhang, Xiaobo. “Characterization of a lysin from deep-sea thermophilic bacteriophage GVE2”. Applied Microbiology and Biotechnology. Mar 2008. Volume 78: p. 635–641. Yurkov V, Beatty, J. Thomas. <http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=16349490>. Appl Environ Microbiol. 1998 January; 64(1): 337–341.
Edited by [Vicky Chen , Vicky Kuo , Ban Lam , Tam Pham , Cassie Tom], students of Rachel Larsen
