Dengue Virus and Dengue Fever
1. Classification
Higher Order Taxa
Virus; ssRNA positive-strand viruses; Flaviviridae; Flavivirus; Dengue virus
2. Introduction
The dengue virus (DENV) is a flavivirus that causes the deadly disease dengue fever. When the virus replicates through retroviral means in the infected hosts (primates), it causes this non-contagious mosquito-borne illness. Dengue fever symptoms present four to six days after being infected by a dengue-infected virus. Symptoms in humans include high fevers and extreme headaches, joint and muscle pain, nausea and vomiting, skin rash (usually becoming visible a few days after onset of fever), and bleeding (1). A patient may go through three stages of a fever including a febrile phase, a critical phase, and the recovery phase. If left untreated and the illness worsens, bleeding may become severe and lead to death. Intense bleeding, although small at first, may highlight the development of the disease into dengue hemorrhagic fever (2). Even though dengue is a short-term illness, a severe infection can be deadly. Although most cases occur in tropical climate, the ability of certain vector species, such as Aedes albopictus, to withstand colder temperatures and its spread across Europe has resulted in dengue fever outbreaks in subtropical regions in recent years (3), making the disease a global public health issue.
There are currently five antigenically different serotypes of DENV: DENV-1, DENV-2, DENV-3, DENV-4, and DENV-5, with DENV-5 being the most recently discovered serotype (4). As each serotype has different antigens, acquired immunity to DENV is serotype-specific, although some cross-serotype immunity can also happen for a certain amount of time (WHO). Even though the four serotypes are varied in their interactions with the host, the symptoms presented are in the same range and results in the same disease. The four serotypes also share the same geographical and ecological niches.
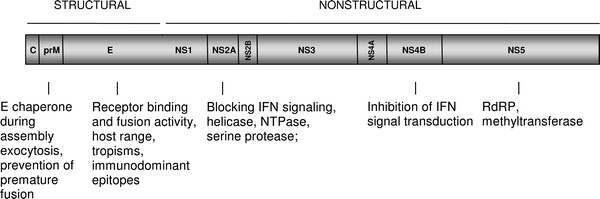
3. Viral Anatomy and Genome Structure
The dengue virus consists of a single-stranded positive sense RNA sequence. The viral RNA genome is divided into two major parts: structural and non-structural. The structural part of the genome encodes three major proteins: C, M, and E (5). All components of the virus are covered by a lipid bilayer (3). The non-structural part of the genome codes for seven non-structural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (5). During infection, the dengue virion attaches to cell receptors. Endocytosis (3,4) occurs and glycoprotein E is activated, allowing the fusion of the viral and the infected cell membranes. Afterwards, viral RNA is reverse-transcribed and replicated (6). The C protein forms the nucleocapsid that packages the newly amplified RNA and transports the capsid to the endoplasmic reticulum and the Golgi. This is also where the M protein is cleaved, resulting in maturation of viral particles (6).
Adapted and revised from Zybert (2011). Genome map of the Dengue Virus.
Due to the poor fidelity of RNA replication in Dengue virus, viral genomes vary greatly from one host to another, and this variation is termed intrahost diversity (7). Intrahost diversity gives rise to interhost diversity, variations of genotypes between hosts on a certain consensus level (8). A study in 2017 sequenced genomes of DENV-3 serotype taken from individuals in Nicaragua. The findings concluded that rapid evolution of DENV-3 inside of hosts comes from pre-existing immunity from patients, among other factors. The study also found an immune-escape strain of the virus, although this strain has relatively severe replication defects (8). Immune-escape strains are strains that will infect a host even though they have already experienced the same infection before.
4. Life Cycle and Replication
The dengue virus proliferates through the sylvatic cycle between humans and mosquitoes. In this cycle, an infected mosquito will spread the disease to a human or primate, then the infected person will be bitten by uninfected mosquitoes and become infected. Vertical transmission from a mother mosquito to its progeny has been shown to exist as well in rare cases (9). Several mosquito species of the Aedes genus are known to be transmission vectors of the dengue virus. A 2017 study compared the numbers and distribution of two Aedes species in Borneo and how they differ in forest and agricultural areas. The difference in abundance between the two areas does not affect the impact of dengue virus on humans (10).
Since, DENV is a retrovirus, it follows the traditional retroviral infection pathway This means turning its RNA into host cell DNA and then making copies from the host machinery. Initially, a viral particle will find a target cell by E protein attachment to a cellular receptor protein of the target (the protein has not been identified as of yet). The virus maintains contact with this receptor and uses endocytosis to enter the cell. After the vesicle is exposed to pH’s lower than physiological pH, the nucleocapsid of the virus is released (9). This leads to the free viral RNA in the cell that is then reverse transcribed into the nucleus of the infected cell. The cell begins to create more nucleocapsids through the use of the endoplasmic reticulum and after this, the golgi bodies further modify the virion through acidification and send the resulting matured virions out of the cell to infect other cells (9).
While in a mosquito vector, DENV follows different replication procedures than in mammalian hosts (figure below). Mosquito protein endoplasmic reticulum kinase (PERK) has been shown to be useful in helping cells survive after the mosquito cell has been infected The PERK proteins in mosquitoes reduce DENV2-induced endoplasmic reticulum stress. Humans and primates do not have this kinase so infection with DENV causes endoplasmic reticulum stress and autophagy to occur in infected cells. The process of autophagy leads to more proliferation of the virus in human cells because it causes the release of more viral particles.
5. Treatment
Treatment for dengue virus does not yet exist, although research is being done for possible ways to stop the infection metabolically. The only treatment is to treat the symptoms according to the way the patient presents them. A study from 2017, reported that a rapid diagnosis combined with supportive treatment is key to treating symptoms and preventing morbidity. A patient had a good response to treatment with hemodialysis, plasma exchange and steroids (12). Aside from treatment, there is also no vaccine that exists to protect individuals from infection. An example of a study from 2009 reveals that loss of nuclear localization signals in DENV C protein can prevent apoptosis in liver cells, since dysfunctional DENVC is unable to interact with apoptotic protein Daxx to trigger cell death (13). As a result, DENVC may serve as a potential drug target to prevent apoptosis and systemic inflammation in dengue fever patients.
6. Current Research and Recommendations
Among those who are infected, most individuals recover from a febrile illness without treatment, since there is no specific treatment. The recommendations for the future are that epidemiologists should continue to understand the burden of the disease, and researchers work together to come up with a possible treatment plan and vaccine to prevent it from spreading. A study from September 2017 shows how inhibiting the PERK signaling pathway resulted in a lighter stress response in a eukaryotic complex. The researchers concluded that PERK signaling is involved in keeping cells alive and could reduce the effects of DENV infected cells (14). This study shows that there are possibilities for treatment, and it could include drugs that inhibit the PERK signaling pathway. The vaccine must produce protective immunity against all homologous serotypes, in order for it to be effective. Protection against heterologous serotypes might be short lived and have different immune responses. Despite the difficulties in creating a vaccine or treatment, countries that are affected should work on prevention of dengue by keeping areas clear of breeding sites (14), which could be done by removal of stagnant water sources.
9. References
1. Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, and Morse AP (2012) Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface 9(75): 2708-2717.
2. Mustafa MS, Ratsogi V, Jain S, and Gupta V (2015) Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med J Armed Forces India 71(1): 67-70.
3. Rodenhuis-Zybert IA, Wilschut J, and Smit JM (2010) Dengue virus life cycle: viral and host factors modulating infectivity. Cell and Molecular Life Sciences 67(16): 2773-2786.
4. Lindenbach BD and Rice CM (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott Williams & Wilkins, Philadelphia), pp. 991–1041.
5. Modis Y, Ogata S, Clements D, Harrison SC (2003) A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U. S. A. 100(12): 6986-6991.
6. Domingo E and Holland JJ (1997) RNA virus mutations and fitness for survival. Annual Review of Microbiology 51: 151-178.
7. Parameswaran P, Wang C, Trivedi SB, Eswarappa M, Montoya M, Balmaseda A, and Harris E (2017) Intrahost selection pressures drive rapid dengue virus microevolution in acute human infections. Cell Host and Microbe 3: 400.
8. Nieto-Ríos, J. F., Álvarez Barreneche, M. F., Penagos, S. C., Bello Márquez, D. C., Serna-Higuita, L. M., & Ramírez Sánchez, I. C. (2017). Successful treatment of thrombotic microangiopathy associated to dengue infection: A case report and literature review. Transplant Infectious Disease: An Official Journal of the Transplantation Society. https://doi.org/10.1111/tid.12824
9. Young KI, Mundis S, Widen SG, Wood TG, Tesh RB, Cardosa J, Vasilakis N, Perera D, and Hanley, KA (2017) Abundance and distribution of sylvatic dengue virus vectors in three different land cover types in Sarawak, Malaysian Borneo. Parasites and Vectors 10: 406.
10. Haddow AD, Guzman H, Popov VL, Wood TG, Widen SG, Haddow AD, Tesh RB, and Weaver SC (2013). First isolation of Aedes flavivirus in the Western Hemisphere and evidence of vertical transmission in the mosquito Aedes (Stegomyia) albopictus (Diptera: Culicidae). Virology 440 (2): 134–139.
11. Screaton G, Mongkolsapaya J, Yacoub S, and Roberts C (2015). New insights into the immunopathology and control of dengue virus infection. Nature Reviews Immunology 15: 745-749.
12. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, and Hay SI (2013) The global distribution and burden of dengue. Nature 496: 504-507.
13. Netsawang J, Noisakran S, Puttikhunt C, Kasinrerk W, Wongwiwat W, Malasit P, Yenchitsomanus P, and Limjindaporn T (2010). Nuclear localization of dengue virus protein is required for DAXX interaction and apoptosis. Virus Research 147(2): 275-283.
14. Hou JN, Chen TH, Chiang YH, Peng JY, Yang TH, Cheng CC, Sofiyatun E, Chiu CH, Chiang-Ni C, and Chen WJ (2017) PERK Signal-Modulated Protein Translation Promotes the Survivability of dengue 2 Virus-Infected Mosquito Cells and Extends Viral Replication. Viruses 9: 262.
15. WHO: Dengue and severe dengue. 2017, April. http://www.who.int/mediacentre/factsheets/fs117/en/
Edited by Douglas Blais, Kathleen Desevin, Aarti Patel, Meagan Sylver, students of Jennifer Talbot for BI 311 General Microbiology, 2015, Boston University.