Dengue transmission
Effects of Pathogen-Vector Interactions on the Transmission of Dengue Virus
A Viral Biorealm page on the family Dengue transmission
Dengue Virus
Dengue virus is the causative agent of both classical dengue fever and the more severe dengue hemorrhage fever and dengue shock syndrome [3]. It is most commonly transmitted by the mosquito vector Aedes aegyptibut can replicate within and be transmitted by other members of the genus Aedes including Aedes albopictus (Figure 2)[4]. There are four different serotypes of dengue virus (DENV 1-4). Infection with one serotype affords life-long immunity to that serotype but only partial (heterologous) immunity to other serotypes for a short period of time post-infection. After this initial, partial immunity the risk of developing dengue hemorrhage fever and dengue shock syndrome upon reinfection by another dengue serotype goes up. This increased risk for severe dengue results from antibody-dependent enhancement of viral infection. Antibodies from previous dengue infection increase the ability of the new strain to infect cells increasing rates of viral replication which results in more severe clinical manifestations of dengue =. Within serotypes dengue viruses are organized by genotypes, then subtypes, then clades, variants, groups and finally strains [3]. The large number of dengue strains in the world combined with only partial crossover immunity to other strains makes concurrent (multiple strain) infections or reoccurring dengue infections possible (just like repeated bouts of the flu), especially in areas that have a high prevalence of dengue virus.
Dengue viruses are now endemic to tropical areas of the world putting about a third of the world’s population at risk of infection. There are estimated to be over one hundred million new infections per year and rising [4,5]. The rising rates of infection are a result of reduced vector control in areas that need it most. As well as the expansion of the geographic range of both vector and virus has also increased the incidence of the disease [6].
The severity of dengue infection also continues to increase. The ratio of dengue hemorrhage fever and dengue shock syndrome cases to classical dengue fever have increased dramatically over the past sixty years. It has become the leading cause of hospitalization and death in children in several endemic countries [6].
Dengue Fever
Although classical dengue is not usually fatal it has very high morbidity, its alternate name is break-bone fever for the severe joint pain during infection. Disease is most common in infants, young children, and adults. Classical dengue manifests itself with a mild to high fever, red rash, debilitating headaches, muscle and joint pain.
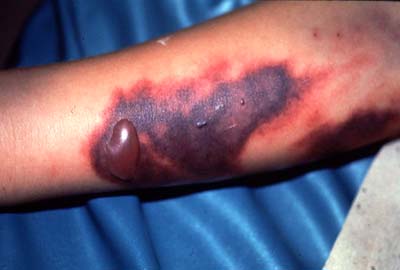
The more severe manifestations of the disease are dengue hemorrhage fever and dengue shock syndrome. Young children and those previously infected with dengue are most at risk for these complications from dengue infection [4]. Dengue shock syndrome results when capillaries leak as result of infection. This leads to edema (swelling of tissue because of fluid escaping the circulatory system), abdominal pain (as a result of the edema) and hypotension (low blood pressure resulting from a drop in total blood volume from fluid loss). Oxygen and nutrients stop reaching the bodies tissues because of inadequate circulation of the blood as a result of these symptoms and this can lead to shock and death [5]. Dengue hemorrhage fever occurs when normal blood coagulation is disrupted by infection. Fever, emesis (vomiting), and hemorrhaging (bleeding) are all symptoms common to this type of dengue [5]. The results of internal hemorrhaging can be seen in the form of tiny red spots (petechiae) or sometimes patches under the skin as well as bloody stool (feces) and bleeding from the gums and nose (Figure 3)[4]. Mortality from these complications can be up to 14% without proper care [3].
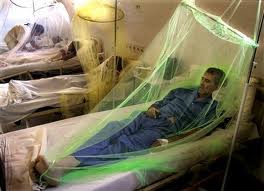
Unfortunately there are no anti-viral drugs for dengue. The only treatment is supportive care in the form of fluid replacement and pain management [4]. With no licensed vaccine, The spread of dengue is currently controlled by halting disease transmission. Controlling transmission on the human side of the viral lifecycle is fairly simple. It involves preventing infected persons from coming in contact with mosquitoes with the use of a mosquito net (Figure 5). The current focus of transmission control has been focused on understanding and finding ways to control the replication and transmission of the virus from vector to host [4].
Life Cycle
Dengue can follow two types of life cycles; sylvatic and epidemic depending on the type of strain. Sylvatic strains are antigenetically different than epidemic strain and mostly maintain transmission between nonhuman primates and forest dwelling Aedes aegypti. Human infection by a sylvatic strain is rare.
Epidemic dengue virus strains infect and replicate within humans and domesticated Aedes species [7]. Epidemic dengue has become endemic in tropical urban slums where poor sanitation and free standing water has allowed the number of mosquito vectors to rise (Figure 1)[3]. High vector density combined with high population density in urban areas has allowed dengue virus to get a strong foothold in many of the world’s over-crowded tropical cities [6].
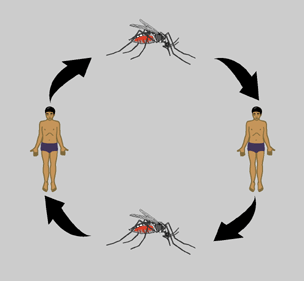
The dengue virus life cycle begins with infection of a human or nonhuman primate host via an infected mosquito vector. Hosts begin to experience symptoms four to seven days post infection with symptoms usually lasting three to ten days after which there is about a three week recovery period [6]. The virus initially replicates in target organs like the liver, spleen, and thymus but eventually makes its way to the lymphatic system where it replicates in lymph tissue and white blood cells. The lymphatic system serves as a gateway to the bloodstream where the virus continues to replicate. Eventually there are very high levels of virus in the blood (viremia) and disease transmission can occur. For a period of about five days, beginning just before symptoms appear the virus is transmissible to a new mosquito vector.
Transmission of dengue virus arises when an uninfected mosquito bites an infected host and takes up virus with its blood meal. The virus replicates in the midgut of the mosquito then infects its hemocoel (body cavity). From the hemocoel the virus eventually makes its way to the salivary glands. Once the salivary glands are infected the mosquito can infect other human hosts and the cycle continues [3]. The length of time required for virus to reach the salivary glands of a mosquito after the ingestion of an infected blood meal is about eight to ten days depending on both viral and host factors [6].
Vector-Host Interactions that Affect Dengue Virus Transmission
The transmission of a particular strain of dengue from vector to host is determined by how infective the virus is in the vector, the level of viral replication in tissues of the vector, and how easily the virus can disseminate from the midgut to the salivary glands [3]. Infectivity, replication, and dissemination are affected by both the genotype of the infecting virus as well as a variety of mosquito host factors that modulate dengue virus infection. Some of the host factors that specifically influence dengue infection and transmission are Aedes vector innate immune system response to viral infection and vector immune response to co-infection of dengue virus and the parasitic bacterium wolbachia. Competitive strain competition between different dengue viruses also affects how well individual strains are transmitted during co-infection and help determine which strains of dengue are maintained in a given host population [3,10,11].
Even slight changes in the pathogen-vector dynamic at the cellular level can effect dengue virus transmission. Minute changes of virus titers in the salivary glands, rate of dissemination of virus from the midgut to the salivary glands as well as a slew of other changes due to pathogen-vector dynamics can have a far reaching impact on the epidemiology of classical dengue as well as severe dengue.
With a better understanding of the involvement of dengue virus genotype and vector host factors in disease transmission it may be possible to manipulate one of these factors to help prevent further spread of dengue fever even without dengue specific drugs or a vaccine.
Competitive Strain Displacement Between Dengue Strains
There are multiple ways the strain prevalent in a given geographic area can change. A given strain can evolve over time until it is antigenetically different enough from the original strain that infection with the parent strain does not confer life-long immunity to this daughter strain making it a new strain [3]. Strains can go extinct in a given geographic area because there are no more susceptible human hosts left. Or a new strain is introduced to the area and takes over as the active disease causing strain because that given population is susceptible to infection. Competitive strain displacement has been documented in a number of cases as the mechanism by which the most prevalent strain in a given area changed [3,12].
Competitive strain displacement occurs when a more virulent strain of virus outcompetes a less virulent strain resulting in a higher frequency of dengue cases due to the more virulent strain. It can occur in either singular infection of a vector where a more virulent strain has higher rates of replication and transmission than the less virulent strain resulting in a higher frequency of dengue infections due to the more virulent strain. Or competitive strain displacement can occur during co-infection of a vector with more than one strain of dengue. The more virulent strain is able to suppress viral replication of the less virulent strain to become the more frequently transmitted strain [3,11].
This phenomenon is one way to explain the increase in the number of severe cases of dengue (dengue hemorrhage fever and dengue shock syndrome) over the past fifty years and the global turnover of dengue strains. The distribution of dengue serotypes in areas with endemic dengue has not changed over the years but the prevalence of different genotypes, subtypes or groups (that have varying levels of virulence in humans) continues to change around the globe. Invasive strains (new to a given geographic area) tend to be more virulent than the native strain already present and therefore are able to become more prevalent than the native strain through competitive displacement [3,11].
In Sri Lanka in the 1980’s an invasive DENV3 strain was introduced that eventually displaced the native DENV3 strain resulting in a dramatic increase in the number of severe dengue cases. Since the 1980’s all four dengue serotypes have been circulating freely in Sri Lanka but only after 1989 was there a sharp rise in cases of dengue hemorrhagic fever and dengue shock syndrome [12].
In both single and co-infection of a vector with dengue viruses there are multiple variables that affect the transmissibility of strain which in turn affects how competitive one strain is relative to another. They include; level of infectivity (how well the virus can infect the vector), rate of dissemination from the midgut to the salivary glands, and replication rate within the midgut and salivary glands. Depending on the strains being compared the infection rate of the vector by an invasive (and usually more virulent strain) is either the same or higher compared to the native strain [3,11]. Faster dissemination rates in single strain infections between invasive and native strains have also been observed. Rate of virus replication within both the midgut and salivary gland are higher in invasive strains as well [3,11].
Vectoral capacity is the frequency at which a mosquito bites and can potentially transmit infection to a human host following ingestion of an infected blood meal. It is determined by a number of factors including length of vector survival and vector competence [11]. Vectoral survival is the length of time the vector (mosquito) lives (and can bite and infect hosts) after infection. Vectoral competence is the capacity of a vector to be infected by, maintain, and transmit a virus. In vectors infected with the more virulent invasive strains vectoral capacity is heightened which increases frequency of transmission and prevalence of the invasive strain. Although the exact cause is unknown, mosquitoes infected with invasive strains are more likely to survive the incubation period of the virus (the first eight to ten days after infection) and go on to transmit dengue virus than mosquitoes infected with native strains. This especially holds true when the overall vector survival rate is low due to environmental factors such as temperature and humidity [11].
In studies of co-infection of vector cells with more virulent and less virulent strains, the more virulent strain was more competitive than the less virulent strain. Co-infection of strains in mosquito cells reduced viral titers of both strains but the level of replication suppression in the more virulent strains was much lower than the level of suppression in the less virulent strains. This effect was most pronounced at high MOIs (initial amount of virus cells were exposed to during infection). High levels of infecting virus increased the competition between strains and there was increased suppression of viral replication seen in the less virulent strain.
Competitive strain displacement also occurs on the human side of the viral life cycle but is not as much of a concern when looking at ways to prevent the spread of more virulent dengue strains because of methods of transmission control already discussed.
Innate Immune Response of Vector
Effects of Wolbachia on Dengue Replication and Transmission in Vector Host
Epidemiologic Consequences of Various Forms of Vector Control
conclusions
References
[1] Genesio, J.. May 21, 2010. “Beware of the Bite” Natural Unseen Hazards Blog. Available: http://naturalunseenhazards.wordpress.com/2010/05/21/recent-outbreak-of-dengue-cases-are-the-first-locally-acquired-cases-in-florida-since-1934/ [Date visited: 10/30/10]
[2] Funchal Camara Municipal. “Monitoring the Yellow Fever Mosquito” Funchal Camara Municipal. Available: http://www.cm-funchal.pt/Cmf/en/Default.aspx?ID=110 [Date visited: 11/1/10]
[3] Hanley, K.A., J.T., Nelson, E.E., Schirtzinger, S.S., Whitehead, C.T., Hanson. 2008. Superior infectivity for mosquito vectors contributes to competitive displacement among strains of dengue virus. BioMed Central Ecology 8(1)
[4] Centers for Disease Control and Prevention. October 28, 2010. “Dengue” Available: http://www.cdc.gov/dengue [Date visited: 10/28/10]
[5] Dengue Fever. 2010. “Dengue Fever Symptoms” denguefeversymptoms.org. Available: http://www.denguefeversymptoms.org/ [Date visited: 10/31/10]
[6] World Health Organization. 2010. “Dengue” Available: http://www.who.int/topics/dengue/en/ [Date visited: 10/27/10]
[7] Pepin, K.M., K.A., Hanley. 2008. Density-dependent competitive suppression of sylvatic dengue virus by endemic dengue virus in cultured mosquito cells. Vector Borne Zoonotic Diseases. 8(6): 821-828
[8] Prima Quality Media. 2010. “gdeng052.gif” Available: http://www.primaquality.com/2008/03/30/combating-of-dengue-hemorrhagic-fever-scourge/cyrcle-transmission-of-dengue-virus/ [Date visited: 10/29/10]
[9] Worldnews Infectious Disease. 2010. “Recovering dengue fever patient” Available: http://wn.com/infectious_disease [Date visited: 10/28/10]
[10] Moreira, L.A., I., Iturbe-Ormaetxe, J.A., Jeffery, G., Lu, A.T., Pyke, L.M., Hedges, B.C., Rocha, S., Hall-Mendelin, A., Day, M., Riegler, L.E., Hugo, K.N., Johnson, B.H., Kay, E.A., McGraw, A.F., Van Den Hurk, P.A., Ryan, S.L., O’Neill. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and plasmodium. Cell. 139: 1268-1278
[11] Anderson, J.T., R., Rico-Hesse. 2006. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. American Journal of Tropical Medicine and Hygiene. 75(5):886-892
[12] Messer, W.B., U.T., Vitarana, K., Sivanathan, J., Elvitgala, L.D., Preethimala, R., Ramesh, N., Withana, D.J., Gubler, A.D, Silva. 2002. Epidemiology of dengue in Sri Lanka before and after the emergence of epidemic dengue hemorrhagic fever. American Journal of Tropical Medicine and Hygiene. 66(6):765–773
Page authored for BIOL 375 Virology, September 2008