Desulfitobacterium hafniense
Description
Members of the species Desulfitobacterium hafniense can be recognized by several characteristics. It is a rod-shaped bacterium with mild curvature. It ranges in size from 2 to 7µm, and has lateral flagella, if present [1]. There are nine strains of D. hafniense, some of which have sequenced genomes [2, 3]. The majority of species in the genus are Gram-positive [3], yet some strains can be Gram-negative, and some strains can form terminal spores [4]. D. hafniense can be found in environments such as soil, wastewater sludges, and freshwater sediments1. It is an anaerobic organism that is commonly isolated from environments polluted by organic halogenated compounds1. D. hafniense is closely related to the Dehalobacter genus [4] and the species Desulfotomaculum orientis [4]. Its large genome makes it versatile in its functions, and contains reductive dehalogenase genes, some of which have been acquired through transposons, indicating Horizontal Gene Transfer [5]. The bacterium usually occurs in singles, pairs, and small chains and grows optimally at 37⁰C [4]. D. hafniense doesn’t seem special at first glance, but it is the contribution to its environment, which makes it an important species.
Significance
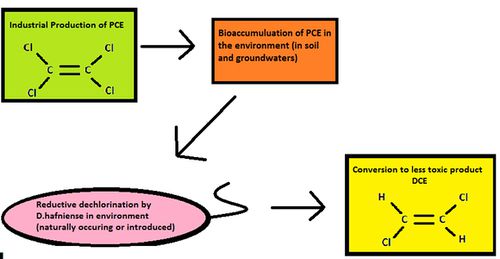
Halogenated hydrocarbons are xenobiotic pollutants used on a large scale as pesticides and degreasing agents [4]. Heavily halogenated anthropogenic chemicals released in the environment are toxic to humans and a wide range of organisms and can affecting ecosystem function. It has been found that there is a relationship between the toxicity of such compounds and the difficulty in degrading them [2]. Perchloroethene (PCE) and trichloroethene (TCE) are common examples of persistent halogenated contaminants because they can only be degraded by very few biological mechanisms [5].Their bioaccumulation due to agricultural applications and industrial production is a reason for concern as they can be present in the environment for long periods of time [6]. Fortunately, there are microorganisms that can degrade these toxic chemicals to less harmful forms. One such organism that carries out metabolic processes to degrade these compounds is the bacterium Desulfitobacterium hafniense.
Metabolism
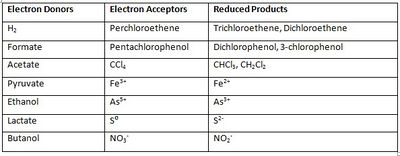
D. hafniense carries out its metabolism through an anaerobic process, which can be very versatile. Some strains are obligate anaerobes, while a few are tolerant to oxygen [3]. It can remove halogen substituents from toxic halogenated organic compounds through a mechanism known as reductive dehalogenation, also called dehalorespiration [1]. Examples of molecules it reduces include PCE, PCB, CCl4 [1], and various chlorophenols, which can be reduced all the way to monochlorophenols [7]. D. hafniense can use a wide range of metals as electron acceptors, and it is also diverse in the electron donors that it uses [3]. The bacterium uses specific enzymes called reductive dehalogenases (Rdhases), the expression of which depends on the presence of certain halogenated substrates. Different Rdhases can degrade several halogenated compounds and are specific to the compound being degraded [1]. D. hafniense doesn’t need prior exposure to the halogenated compounds in order to dechlorinate [4]. The microbe converts TCE to dichloroethene (DCE) via the PceA reductive dehalogenase; however, it is unable to convert it all the way to ethene, unlike the unique species Dehalococcoides ethenogenes [8]. Although its reductive dehalogenation is a beneficial form of metabolism, it can also carry out other forms of respiration, such as nitrate reduction and nitrogen fixation [5]. Its anaerobic respiration conserves energy through the use of electron transport chain and proton motive force [9]. Though D. hafniense is metabolically diverse, it is most commonly known for using halogenated organic compounds as electron acceptors.
Environmental Impact
D. hafniense is seen in a positive light due to its potential to remove pollutants from its environments via its metabolism. It decontaminates areas with a build-up of toxic halogenated compounds, and can convert insoluble metals into soluble ones, which can then be pumped out of the system and recovered. For example, insoluble As(V) can be reduced to As(III) during the dehalogenation process, which will remove arsenic from the system [1].The species has been introduced in soils and sludge containing high levels of pentachlorophenol (PCP), which results in the removal of the contaminant [1], and is an economical method of bioremediation [5]. Another facet in which its metabolism proves useful is in bioreactors, where it decontaminates wastewaters polluted by industrial activities before it is released into larger reservoirs [1]. One consequence of D. hafniense intervention is the accumulation of DCE as an end product [8]. Fortunately, other species such as Dehalococcoides can use anaerobic dechlorination in conjunction with aerobic degradation to oxidize DCE and the carcinogenic intermediate vinyl chloride [10]. D. hafniense can even degrade PCEs that are very water soluble [2]. Specific dehalorespiration molecules can be marked and exploited to encourage cleanup in soils and groundwater [4]. Subsurface soils can host very large populations of D. hafniense, which would give it an advantage at dechlorinating PCE over other bacteria [10]. This would lead to it out-competing other species in those environments. These microscopic organisms can have a profound impact in creating a cleaner and less toxic world around them.
Current Research
There are presently many projects ongoing in order to learn more about how D. hafniense is able to affect its environment and carry out dehalorespiration. In order to detect its presence in potential environments, researchers are examining the 16S rRNA gene sequences of microbial communities, which can determine what species are present [1]. Researchers can then introduce appropriate nutrients to stimulate the community in carrying out its metabolic tasks. The PCR method has also been used as a detection method of the bacterium [6]. Microbiologists are performing genetic analyses to study the function of other genes such as PceT that are present in the same cluster as Rdhase genes [8]. This is being done to better understand the dehalogenation mechanism of Rdhases. What affects the survival of D. hafniense in its environments is an important consideration and depends on a number of factors. These have been found to include organic matter concentration and toxicity of other pollutants [1]. Studies are ongoing to understand how it has physically adapted to face stresses induced by toxic effects of contaminants such as PCE [9]. Other current projects include observing the impact on D. hafniense caused by the structure of surrounding microbial communities, and gene cloning in related genus’ to better understand the structure and function of reductase enzymes [4]. In summary, there is still a lot to be learned about the mechanism of the decontamination processes of D. hafniense.
References
1. Villemur R., Lanthier M., Beaudet R., and Lepine F. 2006. “The Desulfitobacterium genus.” FEMS Microbiol Rev. 30: 706-733.
2. Nonaka H., Keresztes G., Shinoda Y., Ikenaga Y., Abe M., Naito K., Inatomi K., Furukawa K., Inui M., and Yukawa H. 2006. “Complete genome sequence of the dehalorespiring bacterium Desulfitobacterium hafniense Y51 and comparison with Dehalococcoides ethenogenes 195.” J. Bacteriol. 188(6): 2262-2274.
3. Kim SH., Harzman C., Davis JK., Hutcheson R., Broderick JB., Marsh TL., and Tiedje JM. 2012. “Genome sequence of Desulfitobacterium hafniense DCB-2, a Gram-positive anaerobe capable of dehalogenation and metal reduction.” BMC Microbiol. 12: 21.
4. Christiansen N., and Ahring BK. 1996. “Desulfitobacterium hafniense sp. nov., an anaerobic, reductively dechlorinating bacterium.” Int J Syst Bacteriol. 46(2): 442-448.
5. Peng X., Yamamoto S., Vertes AA., Keresztez G., Inatomi K., Inui M., and Yukawa H. 2012. “Global transcriptome analysis of the tetrachlorine-dechlorinating bacterium Desulfitobacterium hafniense Y51 in the presence of various electron donors and terminal electron acceptors.” J Ind Microbiol Biotechnol. 39: 255-268.
6. Smidt H., Akkermans ADL., van der Oost J., and de Vos MW. 2000. “Halorespiring bacteria – molecular characterization and detection.” Enzyme Microb Tech. 27: 812-820.
7. Bisaillon A., Beaudet R., Lepine F., and Villemur R. 2011. “Quantitative analysis of the relative transcript levels of four chlorophenol reductive dehalogenase genes in Desulfitobacterium hafniense PCP-1 exposed to chlorophenols.” Appl Environ Microb. 77(17): 6261-6264.
8. Morita Y., Futagami T., Goto M., and Furukawa K. 2009. “Functional characterization of the trigger factor protein PceT of tetrachloride-dechlorinating Desulfitobacterium hafniense Y51.” Appl Microbiol Biotechnol. 83: 775-781.
9. Prat L., Maillard J., Grimaud R., and Holliger C. 2011. “Physiological adaptation of Desulfitobacterium hafniense strain TCE1 to tetrachloroethene respiration.” Appl Environ Microb. 77(11): 3853-3859.
10. Yoshida N., Asahi K., Sakakibara Y., Miyake K., and Katayama A. 2007. “Isolation and quantitative detection of tetrachloroethene (PCE) – dechlorinating bacteria in unsaturated subsurface soils contaminated with chloroethenes.” J Biosci Bioeng. 104(2): 91-97.