Desulfonatronovibrio hydrogenovorans
Classification
Higher Order Taxa
Bacteria; Proteobacteria; delta/epsilon subdivisions; Deltaproteobacteria; Desulfovibrionales; Desulfohalobiaceae; Desulfonatronovibrio
Species
Desulfonatronovibrio hydrogenovorans
NCBI: Taxonomy
Description and Cell Structure
Desulfonatronovibrio hydrogenovorans, strain Z-7935T (DSM 9292), was first reported in 1994 by Zhilina and Zavarzin and isolated from the sediments of Lake Magadi in Kenya, a soda-depositing alkaline lake, and was later found to extend to soda lakes in Siberia [1]. D. hydrogenovorans is a well-adapted, alkaliphilic, sulfate-reducing bacterium capable of growing in high salinity and extreme alkaline environments [1] Despite being isolated from a hyper saline soda lake, D. hydrogenovorans is a low-salt organism [3]. They are lithoheterotrophic, strictly anaerobic, and sodium-dependent for growth [1].
D. hydrogenovorans closely resembles the typical Desulfovibrio, such as Desulfovibrio desulfuricans and Desulfovibrio magneticus [10]. D. hydrogenovorans contains a gram-negative cell wall structure with a well-developed periplasmic space, and exhibit a curved rod shape similar to Vibrio [1]. They are highly motile utilizing its polar flagella and filamentous appendages for transport and have also been observed to use short spirilla in basic environments [1]. D. hydrogenovorans, which can be found singularly or in pairs, are 0.5μm in diameter and 1.5-2μm in length[1].
D. hydrogenovorans is an obligately sodium-dependent alkaliphile, therefore, it does not have the ability to grow at neutral or acidic pH [1]. The optimal pH for growth is 9.5-9.7 although its maximum pH for growth can reach more than 10[1]. The ideal concentration of NaCl in its environment is 3% (wt/vol) and with 37oC as its optimum temperature for growth, no growth was observed at temperatures above 45oC or below 15oC [1].
Phylogenetics
The genome of D. hydrogenovorans has yet to be sequenced, however the 16s rRNA has been sequenced and the GC content has been determined chemically (48.6 mol%) [1]. Phylogenetic analysis of the 16s rRNA demonstrated that the closest relatives to D. hydrogenovorans are Desulfohalobium retbaense, and Desulfohalobium strain TD3, with 88.7% and 86.6% similarity, respectively [1]. Comparisons between 16s rDNA revealed that D. hydrogenovorans belonged to the delta subclass of the Proteobacteria, and based on its cell structure and metabolic machinery, the name Desulfonatronovibrio hydrogenovorans was proposed [1].
Metabolism
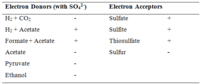
The metabolism of D. hydrogenovorans is very limited to specific electron donors and acceptors [4]. They use hydrogen and formate as its only electron donors, while its only electron acceptors are sulfate, sulfite, and thiosulfate, but not elemental sulfur as it has been shown to inhibit growth. D. hydrogenovorans also has the ability to dismutate (decompose) thiosulfate, sulfite, and sulfur [1], in the absence of electron donors, producing sulfate and sulfide [4]. In the absence of Na+, dismutation of thiosulfate was also seen [1].
D. hydrogenovorans is strictly sodium-dependent for growth as shown by the electroneutral symporter and electrogenic antiporter they possess [4], similar to that of many sulfate-reducing organisms such as Desulfovibrio salexigens [8]. The electroneutral symporter used by D. hydrogenovorans simultaneously transports 2 Na+ and SO42- into the cell for sulfur reduction [4]. The electroneutral symporter relies on a Na+ concentration gradient to drive the intake of SO42- [4]. The electrogenic antiporter is important for maintaining pH homeostasis demonstrated during the growth of D. hydrogenovorans, as their cytoplasm was more acidic than their environment [4]. Although the stoichiometric ratio of H+ transported out of the cell and Na+ transported into the cell is unknown, substantially more protons are transported across for every sodium ion [4]. The protons transported out of the cell by the electrogenic antiporter are coupled with a H+-translocating ATP synthase resulting in ATP synthesis [4]. Unlike D. hydrogenovorans, other alkaliphilic sulfate-reducing organisms, such as Desulfonatronum lacustre, couple ATP conservation to Na+ translocation rather than proton translocation [9]. During carbon dioxide fixation, D. hydrogenovorans utilizes the acetyl-CoA pathway characterized by a key enzyme, CO-dehydrogenase [5]. CO-dehydrogenase acts as a Na+ pump, forming a sodium-motive force [11], which was favored during sulfidogenesis when H2 was used as the electron donor [5]. However, using the Na+ gradient for sulfidogenesis results in the reduction of ATP synthesis by the electrogenic antiporter [4]. Unlike homoacetogenic fermentation of sugar involving energy conservation by substrate level phosphorylation [11], D. hydrogenovorans depends on electron-transport phosphorylation by a chemiosmotic mechanism [5].
Ecology and Significance
D. hydrogenovorans are large hydrogen sinks and can play a significant role in the alkaline lake community [6]. D. hydrogenovorans consume H2 3-4 orders of magnitude greater than hydrogen-utilizing methanogens, resulting in their significant role in hydrogen consumption [7]. Due to the high rates of H2 consumption, they compete with many other H2 consumers such as acetogens, and many other alkaliphilic, sulfate-reducing bacteria [7]. Like D. hydrogenovorans, hydrogen-consuming sulfate-reducing bacteria may be responsible for the extreme alkaline conditions of up to pH 10, due to the secretion of sulfide, a by-product [1]. The by-product H2S, produced by D. hydrogenovorans, is anaerobically oxidized by purple bacteria or aerobically by alkaliphilic thionic bacteria [1], thus D. hydrogenovorans forms the critical link between the carbon and sulfur cycles [6] by utilizing carbon sources and providing reduced sulfur for sulfur phototrophs to oxidize in an anaerobic environment [7]. D. hydrogenovorans can cause problems from the metabolites they produce, such as H2S [13]. They can play a significant role in the decomposition of concrete and metals as a consequence of H2S during biogenic sulfide corrosion [13]. H2S can diffuse into the environment above the water, and O2 from the air can dissolve into these droplets of H2S, resulting in an environment for sulfur oxidizing bacteria to metabolize H2S into H2SO4, a toxic and highly corrosive chemical [13].
References
(1) Zhilina, T. N., Zavarzin, G. A., Rainey, F. A., Pikuta, E. N., Osipov, G. A., and Kostrikina, N. A. “Desulfonatronovibrio hydrogenovorans gen. nov., sp. nov., an Alkaliphilic, Sulfate-Reducing Bacterium.” International Journal of Systematic Bacteriology, 1997, 47(1): 144-149.
(2) Pikuta, E. V., Hoover, R. B., Bej, A. K., Marsic, D., Whitman, W. B., Cleland, D., and Krader, P. “Desulfonatronum thiodismutans sp. nov., a novel alkaliphilic, sulfate-reducing bacterium capable of lithoautotrophic growth.” International Journal of Systematic and Evolutionary Microbiology, 2003, 53: 1327-1332.
(3) Foti, M., Sorokin, D. Y., Lomans, B., Mussman, M., Zacharova, E. E., Pimenov, N. V., Kuenen, J. G., and Muyzer, G. “Diversity, Activity, and Abundance of Sulfate-Reducing Bacteria in Saline and Hypersaline Soda Lakes.” Appl Environ Microbiol, 2007, 73(7): 2093-2100.
(4)Sydow, U., Wohland, P., Wolke, I., and Cypionka, H. “Bioenergetics of the alkaliphilic sulfate-reducing bacterium Desulfonatronovibrio hydrogenovorans.” Microbiology, 2002, 148: 853-860. (5) Pusheva, M. A., Pitryuk, A. V., and Berestovskaya, Y. “Metabolic Peculiarities of the Extremely Alkaliphilic Sulfate-reducing Bacteria Desulfonatronum lacustre and Desulfonatronovibrio hydrogenovorans.” Academician of the Russian Academy of Sciences, 1999.
(6) Jones, B. E., and Grant, W. D. “Microbial diversity and ecology of the Soda Lakes of East Africa.” Atlantic Canada Society for Microbial Ecology, 1999.
(7) Grant, W. D. “Alkaline Environments and Biodiversity.” Encyclopedia of Life Support Systems, 2006.
(8) Kreke, B., and Cypionka, H. “Role of sodium ions for sulfate transport and energy metabolism in Desulfovibrio salexigens.” Archives of Microbiology, 1994, 161(1): 55-61.
(9) Pikuta, E. V., Zhilina, T. N., Zavarzin, G. A., Kostrikina, N. A., Osipov, G. A., & Rainey, F. A. “Desulfonatronum lacustre gen. nov., sp. nov.: a new alkaliphilic sulfate-reducing bacterium utilizing ethanol.” Microbiology, 1998, 67(1): 105-113.
(10) Taylor, James, and R. John Parkes. "The cellular fatty acids of the sulphate-reducing bacteria, Desulfobacter sp., Desulfobulbus sp. and Desulfovibrio desulfuricans." Journal of General Microbiology, 1983, 129(11): 3303-3309.
(11) Ragsdale, S. W., and Wood, H. G. “Acetate Biosynthesis by Acetogenic Bacteria.” The Journal of Biological Chemistry, 1984, 260(7):3970-3977.
(12) Ferry, J. G. “CO dehydrogenase.” Annual Reviews in Microbiology, 1995, 49:305-33.
(13) Barton, L. L., and Guy D. F. "Biochemistry, physiology and biotechnology of sulfate-reducing bacteria." Adv Appl Microbiol, 2009 68: 41-98.