Diphtheria: Difference between revisions
No edit summary |
No edit summary |
||
| Line 24: | Line 24: | ||
====Adherence==== | ====Adherence==== | ||
The exact mechanism of adherence by the pili is not known, but many studies have been conducted to formulate a reasonable proposal. It has been discovered that the two minor pili, SpaB and SpaC, are not only found in the pilus, but are also arranged on the bacterial cell wall in monomeric and heterodimeric forms. The binding of SpA- to pharyngeal epithelial cells can be attributed to these two pili. Since these adhesins are found in two forms, as a fiber and intricate proteins on the cell surface, they can mediate both distant and nearby contacts during the initial adherence of the pathogen and its succeeding colonization [[#References|[7]]]. If either minor pili is absent, then adherence to the host cells is greatly diminished. Little is known about the steps that the SpaD- and SpaH- pili take to adhere to the lung and laryngeal epithelial cells, but it is still postulated that they play an imperative role in the process [[#References|[8]]]. | The exact mechanism of adherence by the pili is not known, but many studies have been conducted to formulate a reasonable proposal. It has been discovered that the two minor pili, SpaB and SpaC, are not only found in the pilus, but are also arranged on the bacterial cell wall in monomeric and heterodimeric forms. The binding of SpA- to pharyngeal epithelial cells can be attributed to these two pili. Since these adhesins are found in two forms, as a fiber and intricate proteins on the cell surface, they can mediate both distant and nearby contacts during the initial adherence of the pathogen and its succeeding colonization [[#References|[7]]]. If either minor pili is absent, then adherence to the host cells is greatly diminished. Little is known about the steps that the SpaD- and SpaH- pili take to adhere to the lung and laryngeal epithelial cells, but it is still postulated that they play an imperative role in the process [[#References|[8]]]. | ||
[[File:Toxin1.jpg|thumb| | [[File:Toxin1.jpg|thumb|200px|right|<i>Toxin Mechanism</i> [http://what-when-how.com/acp-medicine/infections-dues-to-gram-positive-bacilli-part-1/]]] | ||
====Toxin==== | ====Toxin==== | ||
When <i>C. diphtheriae</i> enters the body and adheres to a surface, it will begin to secrete DT. However, there are certain conditions that influence the production of this toxin. For example, the extracellular iron levels in the tissues of the respiratory tract determine when and to what extent DT is released. When levels become very low or are depleted, the bacteria will produce its maximal amounts. This is because iron acts as a corepressor, and will repress the toxin gene when it is present in the extracellular space [[#References|[1]]]. Also, DT will only be produced when it is lysogenized by a specific beta phage. This is because the phage contains a necessary regulatory gene for the structure of the toxin molecule, and the tox genes are found on the phage chromosome instead of the bacterial chromosome. Therefore, both a beta phage and low extracellular iron levels are important for the release of DT [[#References|[1]]]. | When <i>C. diphtheriae</i> enters the body and adheres to a surface, it will begin to secrete DT. However, there are certain conditions that influence the production of this toxin. For example, the extracellular iron levels in the tissues of the respiratory tract determine when and to what extent DT is released. When levels become very low or are depleted, the bacteria will produce its maximal amounts. This is because iron acts as a corepressor, and will repress the toxin gene when it is present in the extracellular space [[#References|[1]]]. Also, DT will only be produced when it is lysogenized by a specific beta phage. This is because the phage contains a necessary regulatory gene for the structure of the toxin molecule, and the tox genes are found on the phage chromosome instead of the bacterial chromosome. Therefore, both a beta phage and low extracellular iron levels are important for the release of DT [[#References|[1]]]. | ||
Revision as of 12:26, 29 July 2015

Etiology/Bacteriology
Taxonomy
| Domain = Bacteria | Phylum = Actinobacteria | Order = Actinomycetales | Family = Corynebacteriaceae | Genus = Corynebacterium | Species = C. diphtheria
Description
Corynebacterium diphtheriae is a gram-positive, non-motile, aerobic, and rod-shaped bacterium that causes diphtheria. There are four main subspecies that have been recognized: C. diphtheriae mitis, C. diphtheriae intermedius, C. diphtheriae gravis, and C. diphtheriae belfanti. C. diphtheriae gravis has the fastest generation time out of the four, allowing it to impose its toxic effects sooner. They can all be characterized as toxigenic or non-toxigenic, or those causing diphtheria and those that don’t, respectively. Diphtheria is an upper respiratory tract infection initially resulting in a sore throat and mild fever, but can progress to other more serious symptoms if not treated [1]. It can also infect the skin when lesions are exposed to the bacteria. Even though there are thousands of reported cases each year, the threat of contracting or succumbing to this illness has dramatically decreased due to advancements in antibiotic treatment and development of vaccinations [2].
Pathogenesis
Virulence factors
C. diphtheriae has two main virulence factors that contribute to its survival in the host. They help the process of adherence in the host and the colonization of the respiratory tract to cause infection.
Pili
The pili found on the surface of C. diphtheriae are beneficial in the adherence to host cells. There are three distinct types of pili expressed including SpaA-, SpaD-, and SpaH- (Spa for sortase-mediated pilus assembly). They are all structurally similar, but have different functions. SpaA- specifically allows for the adherence to pharyngeal epithelial cells, while SpaD- and SpaH- display specificity for binding to lung and laryngeal epithelial cells. There are also two minor pili, SpaB and SpaC proteins, that only bind to pharyngeal cells. The presence of these various pili on C. diphtheriae help it to adhere to certain surfaces, which is necessary for colonization of the host [3].
Toxin
The main virulence factor of C. diphtheriae is diphtheria toxin (DT), an exotoxin, released by the bacteria after entering the human body. DT is classified as an AB toxin because it has two components, one for activation and one for binding. Unlike many other toxins, DT is encoded by a bacteriophage, and it also secreted when extracellular iron levels become low. The major function of the toxin is to enter the cytoplasm and inhibit protein synthesis in susceptible host cells [4]. After the termination of protein synthesis, the production of deoxyribonucleic acid and ribonucleic acid is decreased, and energy metabolism is secondarily affected. All of these factors ultimately lead to cell death [5]. The toxin is carried throughout the body via the bloodstream to reach distant organs, which can occasionally cause paralysis or congestive heart failure [6].
Mechanism
The pathogenesis of C. diphtheriae involves various steps that lead to invasion of host cells, inhibition of protein synthesis, and ultimately cell death. If the bacteria are able to successfully invade and colonize the host, then diphtheria toxin would be released resulting in illness.
Adherence
The exact mechanism of adherence by the pili is not known, but many studies have been conducted to formulate a reasonable proposal. It has been discovered that the two minor pili, SpaB and SpaC, are not only found in the pilus, but are also arranged on the bacterial cell wall in monomeric and heterodimeric forms. The binding of SpA- to pharyngeal epithelial cells can be attributed to these two pili. Since these adhesins are found in two forms, as a fiber and intricate proteins on the cell surface, they can mediate both distant and nearby contacts during the initial adherence of the pathogen and its succeeding colonization [7]. If either minor pili is absent, then adherence to the host cells is greatly diminished. Little is known about the steps that the SpaD- and SpaH- pili take to adhere to the lung and laryngeal epithelial cells, but it is still postulated that they play an imperative role in the process [8].
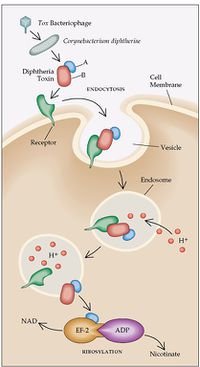
Toxin
When C. diphtheriae enters the body and adheres to a surface, it will begin to secrete DT. However, there are certain conditions that influence the production of this toxin. For example, the extracellular iron levels in the tissues of the respiratory tract determine when and to what extent DT is released. When levels become very low or are depleted, the bacteria will produce its maximal amounts. This is because iron acts as a corepressor, and will repress the toxin gene when it is present in the extracellular space [1]. Also, DT will only be produced when it is lysogenized by a specific beta phage. This is because the phage contains a necessary regulatory gene for the structure of the toxin molecule, and the tox genes are found on the phage chromosome instead of the bacterial chromosome. Therefore, both a beta phage and low extracellular iron levels are important for the release of DT [1].
DT is initially released as a proenzyme, but is then cleaved by bacterial proteases into two fragments, A and B. Fragment A is catalytically active and is the main source of toxicity, while fragment B is rather unstable and has no enzymatic function. Both fragments are used in different ways to allow for entry of DT into the host cell. First, DT will bind to the extracellular epidermal growth factor, which causes a hydrophobic portion of fragment B to form a channel across the host cell membrane allowing fragment A to pass through and reach the cytoplasm [9]. Fragment A then acts as a catalyst to inactivate elongation factor-2 (EF-2), which is necessary for the translation process. A covalent bond is formed between the toxin and EF-2, which disables interaction with RNA during translation, thus, all protein synthesis is halted in that ribosome [5].
Clinical features
The incubation period of C. diphtheriae is between 2-5 days meaning that symptoms will not appear immediately after infection. Initially, a sore throat, fever, nasal discharge, and swollen lymph nodes in the neck will develop. Following this, a thick, gray coating, or pseudomembrane, will grow on the back of the throat making it difficult to swallow and breathe. The pseudomembrane is a congregation of dead tissue that was caused by the diphtheria toxin. If left untreated, this can cause long-lasting breathing complications. Also, if the toxin is in circulation for long periods of time, it can permanently damage certain tissues of the body. For example, it can target heart tissue and lead to myocarditis or it can damage the nerve tissues, especially in the neck, and cause swallowing complications indefinitely [10]. C. diphtheriae can also colonize the skin when an open cut is exposed to it. The lesion will display common symptoms of all bacterial skin infections including redness, swelling, and pain in the region. Also, gray tissues from resulting from dead tissue can surround the laceration as well [11].
Diagnosis
Early diagnosis of this disease is very important because delayed treatment could possibly result in death. Diphtheria can often be confused with other illnesses, so a proper diagnosis in a laboratory is necessary. It is ideal to culture a specimen obtained from the infected patient on blood agar and selective tellurite media. C. diphtheriae reduce the tellurite salts, which result in the development of black colonies on the media making diagnosis of this disease accurate and clear [12].
Treatment
Often, treatment is administered to an infected patient before getting the diagnostic results from a laboratory to increase the chances of complete eradication of the disease. Antitoxin and antibiotics are the main and most effective form of treatment for diphtheria. The antitoxin, which is extracted from horses, targets and neutralizes the DT found throughout the bloodstream to stop the progression of the illness. It is not able to inhibit DT that is already bound to tissues, however. The next common form of treatment is the administration of erythromycin or penicillin, which also targets the toxin and stops the growth of the bacteria. These forms of treatment are both effective in preventing the transmission of diphtheria to other susceptible hosts [10].
Vaccination
There are four vaccines that have been developed to treat diphtheria: DTaP, DT, Td, and Tdap. They were formulated as a combination with other drugs to also treat tetanus and pertussis. DTaP and DT are given to children under the age of seven, while Td and Tdap are administered during adolescence or adulthood. The latter two vaccines are used as boosters and are not given at the same time [13].
Prevention
Receiving the full round of vaccinations for diphtheria is the most effective preventative measure one can take. However, if someone does contract the disease, it is necessary to prevent the transmissions of the disease to others. Isolation of those carrying the disease until they have received a clearing by a medical professional is the best way to prevent the spread of diphtheria. Also, those who come into contact with the bacteria, but aren’t showing any symptoms should also receive medicinal treatment to prevent any possible transmission [14].
Host Immune Response
When C. diphtheria first enters the body when there is a breach in the chemical and physical barriers, the immune response is immediate. Multiple divisions of the immune system will work together to prevent the pathogen from adhering and colonizing any area of the body or to eliminate any established bacteria.
Innate Immune Response
Macrophages residing in the tissues are the first line of defense when C. diphtheriae is introduced into the body. They have receptors on their surface that recognize the pathogen-associated molecular patterns (PAMPs) on the surface of the bacteria. When the two meet, the macrophage phagocytoses the pathogen, and begins to degrade it. While this is occurring, the macrophage is also releasing cytokines, chemokines, and lipid mediators to recruit more cells the site of infection, such as neutrophils, and to induce inflammation. The inflammatory response also acts as a way to draw more immune cells to area to help fight the infection and prevent the colonization of any more bacteria [15].
Adaptive Immune Response
The adaptive immune response is issued after the innate response has occurred, and failed to completely eliminate the pathogen.
T Cell-Mediated Immune Response
T cells can differentiate into three different effector cells: CD8 cytotoxic T cells and then CD4 T Helper I and II cells. Cytotoxic T cells do not have much effect on preventing the spread of C. diphtheria because when Fragment A of the exotoxin enters the cytoplasm and halts protein synthesis, the cell will die. Cytotoxic T cells recognize viral peptides on a bacterial surface and induce cell death via the membrane attack complex. If the cell also already died from the toxin then the cytotoxic T cell would not be inhibit the spread of the disease [16].
The two T helper cells are beneficial in the immune response because they attack the circulating exotoxin and the initial infection of C. diphtheriae. T helper I cells help activate the macrophage which will phagocytose the pathogen. This plays a role in the removal of the bacteria that invaded the host. On the other hand, T Helper II cells aid in the differentiation of B cells into immunoglobulins, which is a very important step taken to eliminate the toxin. T Helper II cells are classified as the linking step between the T cell-mediated response to the humoral response [16].
Humoral Immune Response
The humoral response encompasses the transformation of B cell to plasma cells, which then secrete antibodies. It is the antibodies that coat the pathogen and disable it from adhering to and infecting the host cells, a process known as neutralization. Anti-fragment B antibodies, which attack the B component of the diphtheria toxin, are the primary ones responsible for neutralization since it is the B portion that adheres to cell membrane and forms a channel so the toxin can be internalized. If this is blocked by antibodies, then the toxin is not able to properly infect host cells [5].
Damage Response Framework
When C. diphtheriae first enters the body there are no immediate symptoms. It takes approximately 2-5 days for one to notice the infection. Fever, sore throat, and nasal discharge will develop first with an occasional loss of appetite. The lymph nodes in the neck region will continue to swell due to the infection, and around a week after the first exposure to C. diphtheriae a pseudomembrane, or a thick gray covering, will grow on the tonsil, pharynx, larynx, and nasal tissue. This can make breathing difficult temporarily, and if it is not treated permanent breathing complication can develop. In addition, if the toxin gains access to the rest of the body, it can damage heart and nerve tissue that can lead to lasting cardiac and neurological disorders [10]. However, if antibiotics and the antitoxin are administered when the first symptoms appear, the condition of the infected person will begin to improve about 24 hours after the first dose. They will not be infectious after 48 hours while someone who did not receive treatment would be infectious for two to three weeks. If the illnesses progressed before receiving any treatment the recovery period is a little longer. However, if the antibiotics and antitoxin are taken correctly, then most patients should recover completely and not have any lasting complications [17].
References
1 Online Textbook of Bacteriology. Diphtheria
4 Frassetto, L. A.(2006) Corynebacterium infections
10 Center for Disease Control and Prevention. Diphtheria
11 Mayo Clinic Diseases and Conditions: Diphtheria
14 SA Health: Diphtheria-symptoms, treatment, and prevention
Created by Victoria Moss, student of Tyrrell Conway at the University of Oklahoma.
