Dissimilatory metal reduction: Difference between revisions
Natashaj3a (talk | contribs) (Created page with "{{Uncurated}} =Introduction= Dissimilatory metal reduction is a process that is utilized by microbes to conserve energy through oxidizing organic or inorganic electron donors...") |
Natashaj3a (talk | contribs) No edit summary |
||
| Line 8: | Line 8: | ||
==Access to Electron Acceptor== | ==Access to Electron Acceptor== | ||
Fe(III) reductions occur most often in soils, sediments and the subsurface[[#References[[9]]]. The availability of soluble Fe(III) is limited in soil and sediments because the pH is above pH 4; the predominant form is low-solubility [http://en.wikipedia.org/wiki/Iron(III)_oxide-hydroxide Fe(III) (hydr)oxide][[#References[[9]]]. In order to access this insoluble solid-phase of iron, iron-respiring microorganisms utilize soluble [http://toxics.usgs.gov/definitions/electron_shuttles.html electron shuttles] and Fe(III)-chelating compound, and direct electron transfer via outer membrane enzyme, [http://en.wikipedia.org/wiki/Bacterial_nanowires nanowires], or pili[#References[[3]]]. | Fe(III) reductions occur most often in soils, sediments and the subsurface[[#References[[9]]]. The availability of soluble Fe(III) is limited in soil and sediments because the pH is above pH 4; the predominant form is low-solubility [http://en.wikipedia.org/wiki/Iron(III)_oxide-hydroxide Fe(III) (hydr)oxide][[#References[[9]]]. In order to access this insoluble solid-phase of iron, iron-respiring microorganisms utilize soluble [http://toxics.usgs.gov/definitions/electron_shuttles.html electron shuttles] and Fe(III)-chelating compound, and direct electron transfer via outer membrane enzyme, [http://en.wikipedia.org/wiki/Bacterial_nanowires nanowires], or pili[[#References[[3]]]. | ||
[[File: | |||
The location of iron-respiring microbes in a three-dimensional biofilm has an impact on which Fe(III)-acquiring mechanism the microbes are going to engage in. Microbes, that are closer to the mineral surface, preferentially use extracellular membrane-bound enzyme to transfer electrons[[#References[[9]]]. Alternatively, microbes that are embedded in between the matrix, are more likely to transfer electrons through shuttles or nanowires[[#References[[9]]]. | |||
===Fe(III)-reducing Microorganisms=== | |||
There are a number of microorganisms that are able to reduce Fe(III), for instance, [http://en.wikipedia.org/wiki/Geobacter Geobacter metallireducens] and [http://en.wikipedia.org/wiki/Shewanella_putrefaciens Shewanella putrefaciens][[#References[[6]]]. | |||
[[File:Aerobic degradation of TCE.jpg|thumb|400px|right|]] | [[File:Aerobic degradation of TCE.jpg|thumb|400px|right|]] | ||
Revision as of 21:11, 13 December 2012
Introduction
Dissimilatory metal reduction is a process that is utilized by microbes to conserve energy through oxidizing organic or inorganic electron donors and reducing a metal or metalloid. Microbial metal reduction enables organisms to create electrochemical gradients, which provides the chemical energy required for growth. Metal reducing microorganisms are becoming a research focus due to their potential to facilitate bioremediation in areas that are contaminated with heavy metals or radionuclides. Furthermore, these organisms are integral for the development of microbial fuel cell. The mechanisms of dissimilatory metal reduction are significant in order to exploit its detoxifying and electric-generating advantage.
Fe(III) Reduction
Access to Electron Acceptor
Fe(III) reductions occur most often in soils, sediments and the subsurface[[#References9]. The availability of soluble Fe(III) is limited in soil and sediments because the pH is above pH 4; the predominant form is low-solubility Fe(III) (hydr)oxide[[#References9]. In order to access this insoluble solid-phase of iron, iron-respiring microorganisms utilize soluble electron shuttles and Fe(III)-chelating compound, and direct electron transfer via outer membrane enzyme, nanowires, or pili[[#References3].
[[File:
The location of iron-respiring microbes in a three-dimensional biofilm has an impact on which Fe(III)-acquiring mechanism the microbes are going to engage in. Microbes, that are closer to the mineral surface, preferentially use extracellular membrane-bound enzyme to transfer electrons[[#References9]. Alternatively, microbes that are embedded in between the matrix, are more likely to transfer electrons through shuttles or nanowires[[#References9].
Fe(III)-reducing Microorganisms
There are a number of microorganisms that are able to reduce Fe(III), for instance, Geobacter metallireducens and Shewanella putrefaciens[[#References6].
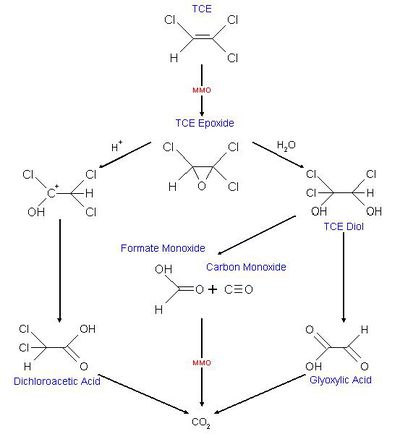
Some dehalorespiring organisms are capable of degrading PCE, TCE and CT into non-toxic compounds. Degradation of PCE is only known to happen through reductive dechlorination and only under anaerobic condition. TCE is, unlike PCE, able to be degraded under aerobic conditions. This can happen through cometabolism. In co-metabolism a compound is transformed by an organism that doesn’t use the compound as an energy or carbon source and reducing power is not provided. The organism relies on another compound to serve as an energy and carbon source [3] . Methanotrophic organisms grow on methane as a primary substrate and oxygen but some are also able to degrade TCE as a secondary substrate. This is because of nonspecific enzymatic activity of enzymes (methane monooxygenase, MMO) involved in degradation of the primary substrate. The degradation of TCE serves no beneficial purpose for these organisms. It generates an epoxide(cf. figure 1) which is transported out of the cell and here other heterotrophic organisms bring about the transformation into non-toxic compounds resulting in the formation of CO2. Several factors inhibit the aerobic degradation of TCE here among the concentration of contamination, the pH and the temperature. Because both TCE and methane bind to the same site in MMO competition between growth substrate and non-growth substrate also seems to limit degradation of TCE [3].
Dehalogenation
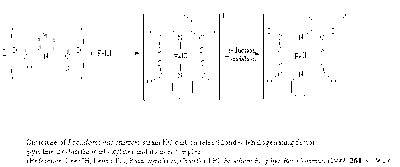
The bacterium Pseudomonas stutzeri strain KC can dehalogenate CT into carbon dioxide and chlorine without producing the toxic intermediate chloroform (CCl3H). This bacterium is originally isolated from an aquifer in Seal Beach in California. It is dependent on anaerobic conditions and in iron-limited media this bacterium produces and secretes a chelator called pyridine-2,6 (bis)thiocarboxylate (PDTC cf. figure 2.) [5]. When PDTC is in contact with a broad range of cell components it turns into a reduced form (the iron in the complex is reduced) and this is essential for its extracellular activity. PDCT has to be in a complex with copper in order for the fast turnover rate of CT into CO2. This complex functions both as a reactant and a catalyst in the reaction. When Pseudomonas stutzeri is in environments were nitrate is present as the electron acceptor a more rapid production of PDTC is observed [6].
Denitrification
In many agricultural areas in North America the nitrate concentrations exceed the standards. Denitrifying organisms are capable of using nitrate or nitrite as terminal electron acceptors thereby removing the excess of nitrogen from the environment. The organism Methylomirabilis oxyfera is an example of such an organism. This denitrifying bacterium is special in that it doesn’t have the gene encoding nitrous oxide reductase, the protein that converts N2O to N2. Instead they harbor an operon which encodes the complete methane monooxygenase complex. This enables it to oxide methane in an aerobic pathway [1]. The mechanism takes advances of the oxidation of methane to drive denitrification. They do so by producing oxygen from nitrite via nitrite oxide (thereby bypassing the intermediate nitrous oxide) and then use this oxygen to oxide methane in an anaerobic environment. This is called nitrite dependent anaerobic methane oxidation. The overall redox reaction is 3CH4 + 8NO2- + 8H+ -> 3CO2 + 4N2 + 10 H2O. In this way the organism uses the potent greenhouse gas methane and reduces nitrite thereby contributing to the removal of excess N-compounds in groundwater [1].
Treatment technologies
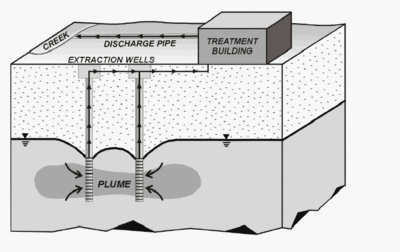
Contamination of groundwater can lead to severe health problems and environmental changes if left untreated. Bioaugmentation is a widespread biological technique used in the removal of chlorinated compounds. By introducing natural electron donors that are helpful in the removal of halogenated compounds into the groundwater the growth of dehalorespiring organisms can be favored. Optional conditions for dehalogenation are provided without any engineering steps taken [7]. Pump and treat method is also one of the most used groundwater remediation techniques. Removal of contaminated groundwater from soil with the use of pumps followed by subsequent remediation at the surface helps overcome the persistence of the pollutants (cf. figure 3). It is typically biological or chemical treatments that remove the pollutants. This method is costly and slow however and some contaminants cannot be removed because they stick to soil and rocks or are not sufficient water soluble [4].
References
(1) Luesken, F. a, van Alen, T. a, van der Biezen, E., Frijters, C., Toonen, G., Kampman, C., Hendrickx, T. L. G., et al. (2011). Diversity and enrichment of nitrite-dependent anaerobic methane oxidizing bacteria from wastewater sludge. Applied microbiology and biotechnology, 92(4), 845–54. doi:10.1007/s00253-011-3361-9
(2) Mahler, R. L., Colter, A., & Hirnyck, R. (2007). Nitrate and Groundwater. University of Idaho Extension.
(3) Peterson, B. C. (1999). Aerobic Degradation of Trichloroethylene. Brigham Young University.
(4) Semkiw, E. S., & Barcelona, M. J. (2011). Field Study of Enhanced TCE Reductive Dechlorination by a Full-Scale Whey PRB, (1), 68–78. doi:10.1111/j1745
(5) Sepúlveda-Torre, L., Huang, A., Kim, H., & Criddle, C. S. (2002). Analysis of regulatory elements and genes required for carbon tetrachloride degradation in Pseudomonas stutzeri strain KC. Journal of molecular microbiology and biotechnology, 4(2), 151–61. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11873910
(6) Smith, L. H., Yang, Y., & De-fg-er, D. O. E. G. N. (2003). Biodegradation of chlorinated solvents: Reactions near DNAPL and enzyme functions, (70063), 1–15.
(7) T. Wilson James. (n.d.). Remediation Apparatus and Method for organic contamination in soil and groundwater.pdf.