Ebola virus entry into host cells: Difference between revisions
Hamdallahi (talk | contribs) No edit summary |
No edit summary |
||
| (24 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==Introduction== | ==Introduction== | ||
<br>By Issam Hamdallah<br> | <br>By Issam Hamdallah<br> | ||
[[File:Ebola | [[File:Colorful Ebola.jpg |thumb|400px|right|Electron micrograph of Ebola virus. http://jdcrighton.com/author/jd-crighton/]] | ||
Ebola viruses belong to the family | Ebola viruses belong to the family Filoviridae. There are three known genuses of filovire: Ebolavirus, Marburgvirus and Cuevavirus. There are five known species of Ebolavirus: Bundibugyo Ebolavirus (BDBV), Zaire Ebolavirus (EBOV), Reston Ebolavirus (RESTV), Sudan Ebolavirus (SUDV), and Taï Forest Ebolavirus (TAFV). The virus got its name from the Ebola river, the river that runs near the discovery site in Sub-Saharan Africa. Species such as Zaire Ebolavirus (EBOV) cause hemorrhagic fever and have up to a 90% mortality rate in humans. Due to the very high mortality rate, this virus is of great concern to scientists today and is considered to be a risk group 4 pathogen. Even species such as Reston Ebolavirus, which causes illness in infected non-human primates but not in humans, are still of great concern because there is a chance that the virus could mutate and become pathogenic to humans (Weingartl et al. 2012). Moreover, since there is no effective vaccine, treatment, or cure for the virus, the virus could potentially be used as a bioterrorism agent. For these reasons, access to the virus is strictly regulated. | ||
The natural reservoir of the virus is still unknown. The virus is believed to have originated in fruit bats, but that has not been confirmed. Outbreaks of | The natural reservoir of the virus is still unknown. The virus is believed to have originated in fruit bats, but that has not been confirmed. One theory is that the virus was present in the saliva of fruit bats and primates that ate the fruits which the fruit bats had come into contact with. Scientists believe Ebolavirus does not infect fruit bats because they have developed an immune response. However, it is deadly in primates. Large percentages of ape populations have been wiped out due to outbreaks of the virus. Outbreaks of Ebolavirus tend to occur in places like Sub-Saharan Africa where the consumption of “bushmeat”- such as primates or bats- is commonplace. This is strong evidence that the virus infects the human population first through animal-human contact, then through human-human contact. | ||
It is important to understand how Ebolavirus enters a host cell since that is the first step in pathogenesis. Ebolavirus is harmless until it enters the host cell, hijacks transcription and translation and begins replicating inside. Understanding how Ebolavirus enters a host cell is crucial in the search for a vaccine, or cure. Developing a means that would prevent Ebolavirus uptake into a host cell would be an effective basis for the development for a vaccine or cure; therefore, it is necessary that researchers understand this process. | |||
==Transmission== | ==Transmission== | ||
Ebolavirus is transmitted through the blood, the exchange of bodily fluids, contact with infected humans, and | Ebolavirus is transmitted through the blood, the exchange of bodily fluids, contact with infected humans, and animals such as primates, fruit bats, and pigs. Consumption of “bushmeat” is just one way that Ebolavirus infects the human population. Unprotected sex, the sharing of needles, and burial practices that involve excessive physical contact with infected corpses are other means by which the virus is transmitted. There is also evidence that EBOV is transmitted from pigs to non-human primates via aerosols (Weingartl et al. 2012). This means that EBOV could potentially be transmitted from infected animals to humans similarly, which demonstrates how dynamic Ebolavirus can be. | ||
Fortunately, outbreaks of ebola have been restricted to relatively rural areas where the population of people is lower and less dense that that of urban areas. The location of the outbreaks, quick death rate, and effective quarantine methods taken by global health officials have prevented Ebolavirus from becoming a global pandemic. The greatest number of people that have died in a given outbreak is 318 people. This was seen in the first outbreak of Ebolavirus in the Democratic Republic of the Congo, formerly know as Zaire, in 1976. Since then, the amount of deaths caused by Ebola have declined, yet the percent fatality rate is still very high. | |||
==Symptoms== | ==Symptoms== | ||
Initial symptoms of Ebolavirus include headache, muscle aches, fever, and fatigue. These symptoms are very similar to other common diseases in Sub-Saharan Africa such as malaria. One theory of how EBOV initially spread is that a person infected with EBOV showing symptoms similar to malaria, received a malaria vaccine at a local missionary outpost (Preston, 1994). The same needle used to give him that vaccine, was used to give others the same vaccine and a lethal amount of EBOV. Soon after initially falling ill, symptoms more specific to EBOV began to surface as the virus began replicating inside of the host. Symptoms of more advanced Ebolavirus infection include vomiting and damage to the lungs, spleen, liver, and blood vessels- all of which result in excessive internal and external bleeding. | |||
Those infected with EBOV usually die within days of infection. Hemorrhagic fever is specific to RNA viruses such as ebola, and is know to cause fever, bleeding, shock and death. Hemorrhagic fever attacks endothelial cells which are found in the interior surface of blood vessels and serve a number of functions. Endothelial cells preform essential bodily functions such as clotting, repairing of damaged organs, and maintaining the overall structural integrity of the blood vessels. Ebolavirus impairs these functions in infected persons. The failure to repair other parts of the body damaged by the virus, the inability to clot blood in order to stop bleeding, and the overall loss of the structural integrity of the blood vessels results in a number of responses that accelerate the death rate in humans. One of the ways that the body responds to the destruction of endothelial cells and the subsequent decrease in blood pressure is by going into shock and “bleeding out”. When this occurs, the infected person’s body shakes violently while simultaneously bleeding out of virtually every orifice in their body. Killing off the host so quickly is not ideal for Ebolavirus since the virus requires a host to survive and replicate, but by going into shock while simultaneously bleeding out, the virus is able to increase its chances of infecting another host. | |||
Strains of Ebolavirus that have not been found to be pathogenic in infected humans include Taï Forest Ebolavirus (TAFV), which was first discovered in China, and Reston Ebolavirus (RESTV), which was isolated from monkeys in the Philippines. These two strains of Ebolavirus are very lethal to nonhuman primates, but not to humans. Strains of Ebolavirus originating in Sub-Saharan Africa such as Bundibugyo Ebolavirus (BDBV), Zaire Ebolavirus (EBOV), and Sudan Ebolavirus (SUDV) are much more deadly in humans. | |||
==Structure== | ==Structure== | ||
Ebolavirus possesses a single-stranded, non-segmented, negative RNA strand genome that contains seven genes: NP, VP35, VP40, GP, VP30, VP24 and L. Ebolavirus contains its genetic information in a nucleocapsid. Once Ebolavirus has successfully entered a host cell, it hijacks the transcription and translation of the host. Ebolavirus can infect an array of host cells which makes the virus a very successful infectious agent. The diameter of Ebolavirus is a consistent 80 nm, but the length ranges from 600 to 1400 nm (Aleksandrowicz et al. 2011). | [[File:Ebola Structure.gif|thumb|400px|right|Figure 1: Ebola virus structure http://www.stanford.edu/group/virus/filo/class.html]] | ||
Ebolavirus possesses a single-stranded, non-segmented, negative RNA strand genome that contains seven genes: NP, VP35, VP40, GP, VP30, VP24, and L. Ebolavirus contains its genetic information in a nucleocapsid. Once Ebolavirus has successfully entered a host cell, it hijacks the transcription and translation of the host. Ebolavirus can infect an array of host cells which makes the virus a very successful infectious agent. The diameter of Ebolavirus is a consistent 80 nm, but the length ranges from 600 to 1400 nm (Aleksandrowicz et al. 2011). Ebolavirus possesses a lipid membrane with glycloproteins on the surface that spike in order for the virus to attach to its host. Inside of the lipid membrane is the viral capsid which encloses the viral RNA (Figure. 1). | |||
==Entry into host cell== | ==Entry into host cell== | ||
EBOV uses macropinocytosis, or clathrin-mediated endocytosis in order to gain access into target host cells. Macropinocytosis is normally used to incorporate larger virus particles into the host cell, whereas clathrin-mediated endocytosis is used to incorporate smaller virus particles into the host cell. Macropinocytosis is a process in which the Eukaryotic host cells form macropinosomes, segments of plasma membranes that extend out from the cell approximately 0.2-10 µm, in order to incorporate the virus into the cell. The formation of macropinosomes | [[File:Figure. 4.png |thumb|300px|right| Figure 2: Ebola and Marburg growth in infected mouse cells. http://au8dt3yy7l.scholar.serialssolutions.com/?sid=google&auinit=JE&aulast=Carette&atitle=Ebola+virus+entry+requires+the+cholesterol+transporter+Niemann-Pick+C1&id=doi:10.1038/nature10348&title=Nature+(London)&volume=477&issue=7364&date=2011&spage=340&issn=0028-0836]] | ||
[[File:Figure 5.png |thumb|300px|right|Figure 3: Proposed model for Ebola virus entry into host cell. http://au8dt3yy7l.scholar.serialssolutions.com/?sid=google&auinit=JE&aulast=Carette&atitle=Ebola+virus+entry+requires+the+cholesterol+transporter+Niemann-Pick+C1&id=doi:10.1038/nature10348&title=Nature+(London)&volume=477&issue=7364&date=2011&spage=340&issn=0028-0836]] | |||
EBOV uses macropinocytosis, or clathrin-mediated endocytosis in order to gain access into target host cells. Macropinocytosis is normally used to incorporate larger virus particles into the host cell, whereas clathrin-mediated endocytosis is used to incorporate smaller virus particles into the host cell. Macropinocytosis is a process in which the Eukaryotic host cells form macropinosomes, segments of plasma membranes that extend out from the cell approximately 0.2-10 µm, in order to incorporate the virus into the cell. The formation of macropinosomes occurs spontaneously, as a result of the activation of various growth factors, or simultaneously with the intake of cellular molecules or extracellular fluid. | |||
Ebolavirus- like particles (EBOV-VLPs), the particles used by Aleksandrowicz et al. 2011 in their study of Ebolavirus entry into host cells, reach a length as long as 2 µm, but stimulate the formation of vesicles approximately 0.5 - 3.5 µm in diameter. EBOV-VLPs enter the protruding segment of the membrane after which the membrane encloses around the EBOV-VLP forming a vesicle and incorporates the virus into the cell. Evidence for this type of activity is demonstrated by the unusually high amount of actin ruffles located on the parts of the eukaryotic plasma membrane with a high concentration of EBOV-VLPs in close proximity. Cells treated with EBOV-VLPs spawned an increase in the number of vesicles formed by eukaryotic cells. Cells that were not treated with EBOV-VLPs did not see any change in the formation of vesicles. The formation of the vesicles via macropinocytosis occurs as a result of actin-mediated ruffle formation, which is activated by the presence of EBOV-VLPs. | |||
Further evidence that EBOV-VLPs use macropinocytosis to gain access to target host cells is seen when drugs are used to inhibit signals and mechanisms required for the host cell to incorporate the EBOV-VLPs via macropinocytosis. Latruncluin A, a toxin that inhibits the binding of actin polymerization, 5(N-Ethyl-N-isopropyl)-amilorid, which inhibits an Na+/H+ antiporter, and wortmannin, a toxin that inhibits PI14-kinase, were the drugs used to inhibit macropinocytosis. In each of the drug treatments, the relative infection rates experienced a significant decline, which means that the EBOV-VLPs were not successfully incorporated into the cells. This is evidence that macropinocytosis is an essential means by which EBOV-VLPs cause infections in host eukaryotic cells. | |||
Another means by which EBOV-VLPs gain access to host cells is clathrin-mediated endocytosis. Clathrin is a protein that is important for vesicle formation. Clathrin-mediated endocytosis is a process by which EBOV-VLPs are engulfed into host cells. An invagination in the membrane of the host cell coated with clathrin proteins is formed and the EBOV-VLPs attach to the receptors on the surface. Once the EBOV-VLPs have attached to the surface, the host membrane encloses upon the EBOV-VLPs and forms a vesicle which carries the EBOV-VLPs within the cell. Eukaryotic cells with depressed levels of clathrin demonstrated a reduction of EBOV-iVLP- or infection by EBOV-VLPs- up to about 25%. This is evidence that clathrin-mediated endocytosis is an essential part of the uptake of EBOV-VLPs into eukaryotic host cells, yet it is not the primary means by which Ebolavirus is taken into the host cell. | |||
Numerous factors specific to EBOV are necessary in order for Ebolavirus to gain access into a host cell. One of the components that has been found to be required in order for EBOV to enter a host cell is EBOV envelope glycoproteins (GP). EBOV GP aids in the binding of EBOV to the host cell by binding to surface receptors on the host cell (Lee and Saphire, 2009). EBOV GP is the only protein expressed by EBOV on the surface membrane of the virus. Glycoproteins spike which allows them to attach to the surface of host cells. In a paper published in 2009, Lee and Saphire proposed five cellular factors- DC-SIGN/L-SIGN, LSECtin, hMGL, β-integrins, and Tyro-3 family receptors- that may be required for attachment to the host cell surface, but none of the cellular factors turned out to be required for viral entry. | |||
The data published by Lee and Saphire in 2009 laid the foundation for Carette et al. (2011) to find that Niemann-Pick C1 (NPC1), a cholesterol transporter, is required for Ebola entry into the host cell. Lee and Saphire, 2009, found that the entry of Ebolavirus is facilitated by viral glycoproteins (GP). Glycoproteins attach filoviruses to the host’s surface, send the viruses to endosomes, and aid in the fusion between the virus and membranes of the endosomes. Carette et al. 2011 created mutations in the homotypic fusion and vacuole protein-sorting (HOPS) complex, which aids in the fusion of endosomes to lysosomes. They also created mutations in the endosomal and lysosomal cholesterol transporter protein NPC1 in order to determine both of their involvementt in Ebola virus entry. It was determined that the NPC1 cholesterol transporter, which is found in the lysosomal membrane, is required for Ebolavirus infection of the host cell (Figure. 2). In that experiment, the viral growth yield of both Ebolavirus (EboV) and Marburgvirus (MarV) with an NPC1 knockout was compared to the viral growth yield of wildtype Ebolavirus and Marburgvirus. There was no difference in the viral growth yield of either species of virus, but viruses with the NPC1 knockout did not grow. NPC1 plays an important role in the entry of the virus and the release of the virus from the vacuole into the cytoplasm of the host (Figure. 3). The HOPS complex was found to be an important component in the process of viral infection, but it is not essential. | |||
Taken all together, Ebolavirus is brought into host cells via endocytosis. There are different types of endocytosis that host cells utilize in order to bring the virus inside of the cell. Macropinocytosis is once way in which Ebolavirus is taken into a host cell. In this process, ruffled segments of the host’s plasma membrane protrude outward from the cell and form invaginations where the virus utilizes glycoproteins in order to attach to the surface of the plasma membrane. Next, the membrane closes in on the virus and, along with other molecules and extracellular fluids, forms a vesicle that is brought into the cell. Once the vesicle is inside of the cell, the NPC1 cholesterol transporter mediates the fusion of the virus with endosomes and lysosomes, after which the virus may leave its vesicle and begin replication. | |||
Clathrin-mediated endocytosis is the other means by which Ebolavirus enters the host cell. This process is very similar to macropinocytosis in that the plasma membrane forms invaginations that engulf the cell. However, clathrin-mediated endocytosis is different in that proteins on the surface of the host’s surface, and in particular clathrin, facilitate the attachment of the virus to the host’s cell surface. Glycoproteins are still used to attach the virus to the cell surface, and the NP-C1 cholesterol transporter still facilitates the fusion of the virus with endosomes and lysosomes and still allows the virus to escape into the cytoplasm. Without the NPC1 cholesterol transporter, Ebolavirus cannot leave the vesicle in order to replicate and cause infection in other cells. | |||
Entry of virus particles is the first step of viral infection; therefore, understanding the processes and mechanisms involved are crucial for treatment, and in the development of a vaccine or cure. Viral entry into a host cell is a complicated process that makes the development of a vaccine very difficult. For example, if the NPC1 cholesterol transporter were knocked out, Ebolavirus would not be able to replicate, thus, the virus could not cause infection. The problem is that the host cannot survive without this protein. Nonetheless, all of the components involved in viral entry into the host cell are a valid means by which a vaccine may be developed, but in order to do so, researchers have to figure out how to develop it without damaging cellular components, or altering processes that would be harmful to the host. | |||
==Vaccines== | ==Vaccines== | ||
To date, there is no form of treatment, cure, or vaccine for | To date, there is no form of treatment, cure, or vaccine commercially available for Ebolavirus infection. Carette et al. 2011 proposed a means for the development of potential antifilovirus pharmaceuticals by inhibiting the NPC1 cholesterol transporter. This has been demonstrated to inhibit EBOV infection in mice, but would block the cholesterol transport pathway; therefore, this form of treatment has not yet been found to be a cure. | ||
In a study conducted by Phoolcharoen et al. (2011), mice were injected with a vaccine that interacts with viral glycoproteins in a way that elicits the production of Ebola immune complexes (EICs). The production of EICs, coupled with Toll-like receptor agonists, which are proteins within the body that stimulate an immune response, resulted in an 80% resistance to the virus in mice. This research presents promising evidence that the development of a vaccine may become a reality in the near future. | |||
More recently, an article written by Ferris Jabr in the April 2014 edition of Scientific American reported that small interfering RNA (siRNA) have been used by researchers at the University of Texas Medical Branch at Galveston to cure monkeys infected with ebola. The siRNA used target a protein in Ebola that inhibits viral replication. The siRNA used only targets the viral proteins; therefore, it does not affect any host cellular components. It is the most promising research to date. | |||
==Conclusion== | ==Conclusion== | ||
Ebolavirus is a rare, yet deadly virus. Although it has not become a global pandemic,it is a very dangerous virus because of its high mortality rate in both humans and non-human primates, lack of an effective vaccine, and ability to mutate. Macropinocytosis and clathrin-mediated endocytosis are the main mechanisms utilized to gain access into target host cells. The method of entry depends primarily on the size of the particle. Macropinocytosis is the primary method for the entry of large viruses, and clathrin-mediated endocytosis uptakes smaller virus particles. Glycoproteins | Ebolavirus is a rare, yet deadly virus. Although it has not become a global pandemic, it is still a very dangerous virus because of its high mortality rate in both humans and non-human primates, lack of an effective vaccine, and ability to mutate. Macropinocytosis and clathrin-mediated endocytosis are the main mechanisms utilized to gain access into target host cells. The method of entry depends primarily on the size of the particle. Macropinocytosis is the primary method for the entry of large viruses, and clathrin-mediated endocytosis uptakes smaller virus particles. Glycoproteins are also required for Ebolavirus to attach and enter the host cell. The Niemann-Pick C1 cholesterol transporter allows the virus to fuse with endosomes and lysosomes. Most importantly, the NP-C1 cholesterol transporter allows the virus to escape into the cytoplasm where the virus can replicate. Ebolavirus entry into the host is the first step towards infection; therefore, understanding the processes is crucial in finding a cure. To this date, there is no vaccine available. Fortunately, Ebola is not responsible for many deaths relative to other diseases such as malaria. The virus has become infamous due to the high fatality rate. Those infected with the more deadly Sub-Saharan strains have a very low chance of survival. | ||
The most recent outbreak of Ebolavirus occurred in Guinea in March of 2014. This outbreak spread to neighboring countries of Liberia and Sierra Leon and was responsible for killing 145 people. This current event informs us that the threat of a global pandemic is still a reality. The virus is know to be carried in animals, and those animals can transmit the virus into the human population of more densely populated regions of the world. Moreover, the virus could mutate into a more deadly and more contagious form of the disease. Outbreaks of Ebola may be overlooked by average American citizens, but the United States Department of Defense is serious about developing a vaccine due the threat of the virus being used as a bioterrorism agent. Tekmira Pharmaceuticals received a $140-million grant to investigate Ebolavirus. As of today, the world is safe from Ebola, but the threat of an outbreak even greater than the 1976 outbreak in Zaire still looms over our heads. | |||
==References== | ==References== | ||
J.E. Carette, M. Raaben, A.C. Wong, A.S. Herbert, G. Obernosterer, N. Mulherkar, A.I. Kuehne, P.J. Kranzusch, A.M. Griffin, G. Ruthel, P. D. Cin, J.M. Dye, S.P. Whelan, K. Chandran, T.R. Brummelkamp. 2011 .Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. Vol. 477: 340-346 | [http://au8dt3yy7l.scholar.serialssolutions.com/?sid=google&auinit=JE&aulast=Carette&atitle=Ebola+virus+entry+requires+the+cholesterol+transporter+Niemann-Pick+C1&id=doi:10.1038/nature10348&title=Nature+(London)&volume=477&issue=7364&date=2011&spage=340&issn=0028-0836 J.E. Carette, M. Raaben, A.C. Wong, A.S. Herbert, G. Obernosterer, N. Mulherkar, A.I. Kuehne, P.J. Kranzusch, A.M. Griffin, G. Ruthel, P. D. Cin, J.M. Dye, S.P. Whelan, K. Chandran, T.R. Brummelkamp. 2011 .Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. Vol. 477: 340-346] | ||
P. Aleksandrowicz, A. Marzi, N. Biedenkopf, S. Becker, T. Hoenen, H. Feldmann, H.J. Schnittler. 2011. Ebola Virus Enters Host Cells by Macropinocytosis and Clathrin-Mediated Endocytosis. Journal of Infectious Diseases. Vol. 204: 957-967 | [http://jid.oxfordjournals.org/content/204/suppl_3/S957.full.pdf+html P. Aleksandrowicz, A. Marzi, N. Biedenkopf, S. Becker, T. Hoenen, H. Feldmann, H.J. Schnittler. 2011. Ebola Virus Enters Host Cells by Macropinocytosis and Clathrin-Mediated Endocytosis. Journal of Infectious Diseases. Vol. 204: 957-967] | ||
H.M. Weingartl, C. Embury-Hyatt, C. Nfon, A. Leung, G. Smith, G. Kobinger. 2012. Transmission of Ebola virus from pigs to non-human primates. Nature. Vol. 811: 1-4 | [http://www.nature.com/srep/2012/121115/srep00811/pdf/srep00811.pdf H.M. Weingartl, C. Embury-Hyatt, C. Nfon, A. Leung, G. Smith, G. Kobinger. 2012. Transmission of Ebola virus from pigs to non-human primates. Nature. Vol. 811: 1-4] | ||
W. Phoolcharoen J.M. Dye, J. Kilbourne, K. Piensook, W.D. Pratt, C.J. Arntzen, Q. Chen, H.S. Mason, M.M. Herbest-Kravlovetz. 2011. Proceeding of the National Academy of Sciences. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Vol. 108(51): 20695–20700 | [http://www.pnas.org/content/108/51/20695.full.pdf+html W. Phoolcharoen J.M. Dye, J. Kilbourne, K. Piensook, W.D. Pratt, C.J. Arntzen, Q. Chen, H.S. Mason, M.M. Herbest-Kravlovetz. 2011. Proceeding of the National Academy of Sciences. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Vol. 108(51): 20695–20700] | ||
[https://www.bcm.edu/departments/molecular-virology-and-microbiology/ebola Baylor College of Medicine. July, 02, 2013. Ebola Virus. (4-12-14)] | |||
[https://www.einstein.yu.edu/news/releases/695/researchers-find-key-used-by-ebola-virus-to-unlock-cells-and-spread-deadly-infection/ Albert Einstein College of Medicine of Yeshiva University. 2014. Researchers Find "Key" Used by Ebola Virus to Unlock Cells and Spread Deadly Infection. (4-12-14)] | |||
[http://www.dailytech.com/Research+Team+Discovers+How+Ebola+Virus+Enters+Replicates+in+a+Host+Cell/article22542.htm Tiffany Kaiser. August 15, 2011. Research Team Discovers How Ebola Virus Enters, Replicates in a Host Cell. (4-12-14)] | |||
Preston, Richard. 1994. The Hot Zone. New York: Random House, 300. | Preston, Richard. 1994. The Hot Zone. New York: Random House, 300. | ||
| Line 81: | Line 89: | ||
Ferris Jabr. 2014. Scientific American. Defeating Nature’s Terrorist- An RNA-based treatment may stop the Ebola virus in its tracks. Scientific American. Vol. 310: 58 | Ferris Jabr. 2014. Scientific American. Defeating Nature’s Terrorist- An RNA-based treatment may stop the Ebola virus in its tracks. Scientific American. Vol. 310: 58 | ||
J.E. Lee, E.O. Saphire. 2009. Future Virology. Ebolavirus glycoprotein structure and mechanism of entry. Vol. 4: 621-635 | [http://www.futuremedicine.com/doi/abs/10.2217/fvl.09.56 J.E. Lee, E.O. Saphire. 2009. Future Virology. Ebolavirus glycoprotein structure and mechanism of entry. Vol. 4: 621-635] | ||
[http://www.washingtonpost.com/blogs/worldviews/wp/2014/03/25/a-brief-history-of-ebola-outbreaks/ Terri Rupar. 3-24-14. A Brief history of Ebola Outbreaks. (4-12-14)] | |||
[ | [http://www.medicinenet.com/ebola_hemorrhagic_fever_ebola_hf/page3.htm C.P. Davis, J.R. Balentine. 4-8-014. Ebola Hemorrhagic Fever (Ebola Virus Disease) (4-12-14)] | ||
[ | [http://www.stanford.edu/group/virus/filo/class.html Tara Waterman. March 1, 1999. Ebola Classification and Taxonomy. (4-12-14)] | ||
Latest revision as of 14:25, 5 October 2015
Introduction
By Issam Hamdallah
Ebola viruses belong to the family Filoviridae. There are three known genuses of filovire: Ebolavirus, Marburgvirus and Cuevavirus. There are five known species of Ebolavirus: Bundibugyo Ebolavirus (BDBV), Zaire Ebolavirus (EBOV), Reston Ebolavirus (RESTV), Sudan Ebolavirus (SUDV), and Taï Forest Ebolavirus (TAFV). The virus got its name from the Ebola river, the river that runs near the discovery site in Sub-Saharan Africa. Species such as Zaire Ebolavirus (EBOV) cause hemorrhagic fever and have up to a 90% mortality rate in humans. Due to the very high mortality rate, this virus is of great concern to scientists today and is considered to be a risk group 4 pathogen. Even species such as Reston Ebolavirus, which causes illness in infected non-human primates but not in humans, are still of great concern because there is a chance that the virus could mutate and become pathogenic to humans (Weingartl et al. 2012). Moreover, since there is no effective vaccine, treatment, or cure for the virus, the virus could potentially be used as a bioterrorism agent. For these reasons, access to the virus is strictly regulated.
The natural reservoir of the virus is still unknown. The virus is believed to have originated in fruit bats, but that has not been confirmed. One theory is that the virus was present in the saliva of fruit bats and primates that ate the fruits which the fruit bats had come into contact with. Scientists believe Ebolavirus does not infect fruit bats because they have developed an immune response. However, it is deadly in primates. Large percentages of ape populations have been wiped out due to outbreaks of the virus. Outbreaks of Ebolavirus tend to occur in places like Sub-Saharan Africa where the consumption of “bushmeat”- such as primates or bats- is commonplace. This is strong evidence that the virus infects the human population first through animal-human contact, then through human-human contact.
It is important to understand how Ebolavirus enters a host cell since that is the first step in pathogenesis. Ebolavirus is harmless until it enters the host cell, hijacks transcription and translation and begins replicating inside. Understanding how Ebolavirus enters a host cell is crucial in the search for a vaccine, or cure. Developing a means that would prevent Ebolavirus uptake into a host cell would be an effective basis for the development for a vaccine or cure; therefore, it is necessary that researchers understand this process.
Transmission
Ebolavirus is transmitted through the blood, the exchange of bodily fluids, contact with infected humans, and animals such as primates, fruit bats, and pigs. Consumption of “bushmeat” is just one way that Ebolavirus infects the human population. Unprotected sex, the sharing of needles, and burial practices that involve excessive physical contact with infected corpses are other means by which the virus is transmitted. There is also evidence that EBOV is transmitted from pigs to non-human primates via aerosols (Weingartl et al. 2012). This means that EBOV could potentially be transmitted from infected animals to humans similarly, which demonstrates how dynamic Ebolavirus can be.
Fortunately, outbreaks of ebola have been restricted to relatively rural areas where the population of people is lower and less dense that that of urban areas. The location of the outbreaks, quick death rate, and effective quarantine methods taken by global health officials have prevented Ebolavirus from becoming a global pandemic. The greatest number of people that have died in a given outbreak is 318 people. This was seen in the first outbreak of Ebolavirus in the Democratic Republic of the Congo, formerly know as Zaire, in 1976. Since then, the amount of deaths caused by Ebola have declined, yet the percent fatality rate is still very high.
Symptoms
Initial symptoms of Ebolavirus include headache, muscle aches, fever, and fatigue. These symptoms are very similar to other common diseases in Sub-Saharan Africa such as malaria. One theory of how EBOV initially spread is that a person infected with EBOV showing symptoms similar to malaria, received a malaria vaccine at a local missionary outpost (Preston, 1994). The same needle used to give him that vaccine, was used to give others the same vaccine and a lethal amount of EBOV. Soon after initially falling ill, symptoms more specific to EBOV began to surface as the virus began replicating inside of the host. Symptoms of more advanced Ebolavirus infection include vomiting and damage to the lungs, spleen, liver, and blood vessels- all of which result in excessive internal and external bleeding.
Those infected with EBOV usually die within days of infection. Hemorrhagic fever is specific to RNA viruses such as ebola, and is know to cause fever, bleeding, shock and death. Hemorrhagic fever attacks endothelial cells which are found in the interior surface of blood vessels and serve a number of functions. Endothelial cells preform essential bodily functions such as clotting, repairing of damaged organs, and maintaining the overall structural integrity of the blood vessels. Ebolavirus impairs these functions in infected persons. The failure to repair other parts of the body damaged by the virus, the inability to clot blood in order to stop bleeding, and the overall loss of the structural integrity of the blood vessels results in a number of responses that accelerate the death rate in humans. One of the ways that the body responds to the destruction of endothelial cells and the subsequent decrease in blood pressure is by going into shock and “bleeding out”. When this occurs, the infected person’s body shakes violently while simultaneously bleeding out of virtually every orifice in their body. Killing off the host so quickly is not ideal for Ebolavirus since the virus requires a host to survive and replicate, but by going into shock while simultaneously bleeding out, the virus is able to increase its chances of infecting another host.
Strains of Ebolavirus that have not been found to be pathogenic in infected humans include Taï Forest Ebolavirus (TAFV), which was first discovered in China, and Reston Ebolavirus (RESTV), which was isolated from monkeys in the Philippines. These two strains of Ebolavirus are very lethal to nonhuman primates, but not to humans. Strains of Ebolavirus originating in Sub-Saharan Africa such as Bundibugyo Ebolavirus (BDBV), Zaire Ebolavirus (EBOV), and Sudan Ebolavirus (SUDV) are much more deadly in humans.
Structure
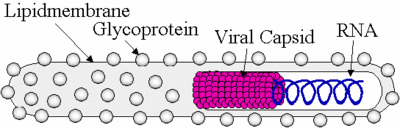
Ebolavirus possesses a single-stranded, non-segmented, negative RNA strand genome that contains seven genes: NP, VP35, VP40, GP, VP30, VP24, and L. Ebolavirus contains its genetic information in a nucleocapsid. Once Ebolavirus has successfully entered a host cell, it hijacks the transcription and translation of the host. Ebolavirus can infect an array of host cells which makes the virus a very successful infectious agent. The diameter of Ebolavirus is a consistent 80 nm, but the length ranges from 600 to 1400 nm (Aleksandrowicz et al. 2011). Ebolavirus possesses a lipid membrane with glycloproteins on the surface that spike in order for the virus to attach to its host. Inside of the lipid membrane is the viral capsid which encloses the viral RNA (Figure. 1).
Entry into host cell
EBOV uses macropinocytosis, or clathrin-mediated endocytosis in order to gain access into target host cells. Macropinocytosis is normally used to incorporate larger virus particles into the host cell, whereas clathrin-mediated endocytosis is used to incorporate smaller virus particles into the host cell. Macropinocytosis is a process in which the Eukaryotic host cells form macropinosomes, segments of plasma membranes that extend out from the cell approximately 0.2-10 µm, in order to incorporate the virus into the cell. The formation of macropinosomes occurs spontaneously, as a result of the activation of various growth factors, or simultaneously with the intake of cellular molecules or extracellular fluid.
Ebolavirus- like particles (EBOV-VLPs), the particles used by Aleksandrowicz et al. 2011 in their study of Ebolavirus entry into host cells, reach a length as long as 2 µm, but stimulate the formation of vesicles approximately 0.5 - 3.5 µm in diameter. EBOV-VLPs enter the protruding segment of the membrane after which the membrane encloses around the EBOV-VLP forming a vesicle and incorporates the virus into the cell. Evidence for this type of activity is demonstrated by the unusually high amount of actin ruffles located on the parts of the eukaryotic plasma membrane with a high concentration of EBOV-VLPs in close proximity. Cells treated with EBOV-VLPs spawned an increase in the number of vesicles formed by eukaryotic cells. Cells that were not treated with EBOV-VLPs did not see any change in the formation of vesicles. The formation of the vesicles via macropinocytosis occurs as a result of actin-mediated ruffle formation, which is activated by the presence of EBOV-VLPs.
Further evidence that EBOV-VLPs use macropinocytosis to gain access to target host cells is seen when drugs are used to inhibit signals and mechanisms required for the host cell to incorporate the EBOV-VLPs via macropinocytosis. Latruncluin A, a toxin that inhibits the binding of actin polymerization, 5(N-Ethyl-N-isopropyl)-amilorid, which inhibits an Na+/H+ antiporter, and wortmannin, a toxin that inhibits PI14-kinase, were the drugs used to inhibit macropinocytosis. In each of the drug treatments, the relative infection rates experienced a significant decline, which means that the EBOV-VLPs were not successfully incorporated into the cells. This is evidence that macropinocytosis is an essential means by which EBOV-VLPs cause infections in host eukaryotic cells.
Another means by which EBOV-VLPs gain access to host cells is clathrin-mediated endocytosis. Clathrin is a protein that is important for vesicle formation. Clathrin-mediated endocytosis is a process by which EBOV-VLPs are engulfed into host cells. An invagination in the membrane of the host cell coated with clathrin proteins is formed and the EBOV-VLPs attach to the receptors on the surface. Once the EBOV-VLPs have attached to the surface, the host membrane encloses upon the EBOV-VLPs and forms a vesicle which carries the EBOV-VLPs within the cell. Eukaryotic cells with depressed levels of clathrin demonstrated a reduction of EBOV-iVLP- or infection by EBOV-VLPs- up to about 25%. This is evidence that clathrin-mediated endocytosis is an essential part of the uptake of EBOV-VLPs into eukaryotic host cells, yet it is not the primary means by which Ebolavirus is taken into the host cell.
Numerous factors specific to EBOV are necessary in order for Ebolavirus to gain access into a host cell. One of the components that has been found to be required in order for EBOV to enter a host cell is EBOV envelope glycoproteins (GP). EBOV GP aids in the binding of EBOV to the host cell by binding to surface receptors on the host cell (Lee and Saphire, 2009). EBOV GP is the only protein expressed by EBOV on the surface membrane of the virus. Glycoproteins spike which allows them to attach to the surface of host cells. In a paper published in 2009, Lee and Saphire proposed five cellular factors- DC-SIGN/L-SIGN, LSECtin, hMGL, β-integrins, and Tyro-3 family receptors- that may be required for attachment to the host cell surface, but none of the cellular factors turned out to be required for viral entry.
The data published by Lee and Saphire in 2009 laid the foundation for Carette et al. (2011) to find that Niemann-Pick C1 (NPC1), a cholesterol transporter, is required for Ebola entry into the host cell. Lee and Saphire, 2009, found that the entry of Ebolavirus is facilitated by viral glycoproteins (GP). Glycoproteins attach filoviruses to the host’s surface, send the viruses to endosomes, and aid in the fusion between the virus and membranes of the endosomes. Carette et al. 2011 created mutations in the homotypic fusion and vacuole protein-sorting (HOPS) complex, which aids in the fusion of endosomes to lysosomes. They also created mutations in the endosomal and lysosomal cholesterol transporter protein NPC1 in order to determine both of their involvementt in Ebola virus entry. It was determined that the NPC1 cholesterol transporter, which is found in the lysosomal membrane, is required for Ebolavirus infection of the host cell (Figure. 2). In that experiment, the viral growth yield of both Ebolavirus (EboV) and Marburgvirus (MarV) with an NPC1 knockout was compared to the viral growth yield of wildtype Ebolavirus and Marburgvirus. There was no difference in the viral growth yield of either species of virus, but viruses with the NPC1 knockout did not grow. NPC1 plays an important role in the entry of the virus and the release of the virus from the vacuole into the cytoplasm of the host (Figure. 3). The HOPS complex was found to be an important component in the process of viral infection, but it is not essential.
Taken all together, Ebolavirus is brought into host cells via endocytosis. There are different types of endocytosis that host cells utilize in order to bring the virus inside of the cell. Macropinocytosis is once way in which Ebolavirus is taken into a host cell. In this process, ruffled segments of the host’s plasma membrane protrude outward from the cell and form invaginations where the virus utilizes glycoproteins in order to attach to the surface of the plasma membrane. Next, the membrane closes in on the virus and, along with other molecules and extracellular fluids, forms a vesicle that is brought into the cell. Once the vesicle is inside of the cell, the NPC1 cholesterol transporter mediates the fusion of the virus with endosomes and lysosomes, after which the virus may leave its vesicle and begin replication.
Clathrin-mediated endocytosis is the other means by which Ebolavirus enters the host cell. This process is very similar to macropinocytosis in that the plasma membrane forms invaginations that engulf the cell. However, clathrin-mediated endocytosis is different in that proteins on the surface of the host’s surface, and in particular clathrin, facilitate the attachment of the virus to the host’s cell surface. Glycoproteins are still used to attach the virus to the cell surface, and the NP-C1 cholesterol transporter still facilitates the fusion of the virus with endosomes and lysosomes and still allows the virus to escape into the cytoplasm. Without the NPC1 cholesterol transporter, Ebolavirus cannot leave the vesicle in order to replicate and cause infection in other cells.
Entry of virus particles is the first step of viral infection; therefore, understanding the processes and mechanisms involved are crucial for treatment, and in the development of a vaccine or cure. Viral entry into a host cell is a complicated process that makes the development of a vaccine very difficult. For example, if the NPC1 cholesterol transporter were knocked out, Ebolavirus would not be able to replicate, thus, the virus could not cause infection. The problem is that the host cannot survive without this protein. Nonetheless, all of the components involved in viral entry into the host cell are a valid means by which a vaccine may be developed, but in order to do so, researchers have to figure out how to develop it without damaging cellular components, or altering processes that would be harmful to the host.
Vaccines
To date, there is no form of treatment, cure, or vaccine commercially available for Ebolavirus infection. Carette et al. 2011 proposed a means for the development of potential antifilovirus pharmaceuticals by inhibiting the NPC1 cholesterol transporter. This has been demonstrated to inhibit EBOV infection in mice, but would block the cholesterol transport pathway; therefore, this form of treatment has not yet been found to be a cure.
In a study conducted by Phoolcharoen et al. (2011), mice were injected with a vaccine that interacts with viral glycoproteins in a way that elicits the production of Ebola immune complexes (EICs). The production of EICs, coupled with Toll-like receptor agonists, which are proteins within the body that stimulate an immune response, resulted in an 80% resistance to the virus in mice. This research presents promising evidence that the development of a vaccine may become a reality in the near future.
More recently, an article written by Ferris Jabr in the April 2014 edition of Scientific American reported that small interfering RNA (siRNA) have been used by researchers at the University of Texas Medical Branch at Galveston to cure monkeys infected with ebola. The siRNA used target a protein in Ebola that inhibits viral replication. The siRNA used only targets the viral proteins; therefore, it does not affect any host cellular components. It is the most promising research to date.
Conclusion
Ebolavirus is a rare, yet deadly virus. Although it has not become a global pandemic, it is still a very dangerous virus because of its high mortality rate in both humans and non-human primates, lack of an effective vaccine, and ability to mutate. Macropinocytosis and clathrin-mediated endocytosis are the main mechanisms utilized to gain access into target host cells. The method of entry depends primarily on the size of the particle. Macropinocytosis is the primary method for the entry of large viruses, and clathrin-mediated endocytosis uptakes smaller virus particles. Glycoproteins are also required for Ebolavirus to attach and enter the host cell. The Niemann-Pick C1 cholesterol transporter allows the virus to fuse with endosomes and lysosomes. Most importantly, the NP-C1 cholesterol transporter allows the virus to escape into the cytoplasm where the virus can replicate. Ebolavirus entry into the host is the first step towards infection; therefore, understanding the processes is crucial in finding a cure. To this date, there is no vaccine available. Fortunately, Ebola is not responsible for many deaths relative to other diseases such as malaria. The virus has become infamous due to the high fatality rate. Those infected with the more deadly Sub-Saharan strains have a very low chance of survival.
The most recent outbreak of Ebolavirus occurred in Guinea in March of 2014. This outbreak spread to neighboring countries of Liberia and Sierra Leon and was responsible for killing 145 people. This current event informs us that the threat of a global pandemic is still a reality. The virus is know to be carried in animals, and those animals can transmit the virus into the human population of more densely populated regions of the world. Moreover, the virus could mutate into a more deadly and more contagious form of the disease. Outbreaks of Ebola may be overlooked by average American citizens, but the United States Department of Defense is serious about developing a vaccine due the threat of the virus being used as a bioterrorism agent. Tekmira Pharmaceuticals received a $140-million grant to investigate Ebolavirus. As of today, the world is safe from Ebola, but the threat of an outbreak even greater than the 1976 outbreak in Zaire still looms over our heads.
References
Baylor College of Medicine. July, 02, 2013. Ebola Virus. (4-12-14)
Preston, Richard. 1994. The Hot Zone. New York: Random House, 300.
Ferris Jabr. 2014. Scientific American. Defeating Nature’s Terrorist- An RNA-based treatment may stop the Ebola virus in its tracks. Scientific American. Vol. 310: 58
Terri Rupar. 3-24-14. A Brief history of Ebola Outbreaks. (4-12-14)
C.P. Davis, J.R. Balentine. 4-8-014. Ebola Hemorrhagic Fever (Ebola Virus Disease) (4-12-14)
Tara Waterman. March 1, 1999. Ebola Classification and Taxonomy. (4-12-14)
