Effects of Nicotine On Cell Cillia: Difference between revisions
Dougherty1 (talk | contribs) |
Dougherty1 (talk | contribs) |
||
| Line 3: | Line 3: | ||
Nicotine(C<sub>10</sub>H<sub>14</sub>N<sub>2</sub>)is a naturally occurring true alkaloid molecule found in tobacco plants. An alkaloid is a class of organic nitrogenous compounds in plants that have prominent physiological impacts on humans. These include, but are not limited to, the inhibition and activation of enzymes, a pronounced effect on nucleic acid and protein synthesis, and effects on membrane structure and cytoskeletal structure and nerve transmission and induction.<ref>[McDaniel, College. “Alkaloids.” Alkaloids, 1999, www2.mcdaniel.edu/Biology/botf99/herbnew/alkaloids.htm.]</ref>Nicotine has gained notoriety in public life due to its addictive nature, which is a direct result of its agonistic behavior in the human brain and nervous system. Nicotine acts as a receptor agonist, a chemical that binds to a receptor and elicits a biological response.<br><br/> Nicotine acts as an agonist to Nicotinic acetylcholine receptors, or nAChRs, a class of receptor polypeptides that respond to the neurotransmitter acetylcholine in the human body. Nicotine competitively binds to these receptors, as it mimics the effects of acetylcholine.<ref>[“The Metabolism of Nicotine .” Metabolism, 2020, www.chm.bris.ac.uk/motm/nicotine/E-metabolisme.html.]</ref><ref>[“Nicotine.” Wikipedia, Wikimedia Foundation, 2 Dec. 2020, en.wikipedia.org/wiki/Nicotine.]</ref><ref>[“Nicotinic Acetylcholine Receptors.” Wikipedia, Wikimedia Foundation, 10 July 2017]</ref>These receptors are found in both nervous systems in the human body, as well as muscles and tissues of the human body. They are the primary receptors at the neuromuscular junction in muscles and are the primary site of nerve and muscle communication. These receptors get their name from nicotine, as nicotine selectively binds to these nicotinic receptors rather than the other receptors in the area.<br><br/>Nicotine is a stimulant, a class of substances that increase physiological and nervous activity in the body. Nicotine stimulates the central nervous system and in turn causes the body to release several neurotransmitters, such as dopamine, acetylcholine, serotonin, and norepinephrine.<ref>[“Nicotine.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/Nicotine.]</ref> However, nicotine only causes a brief uptick in neurotransmitter activity and presence; and the constant activation of these receptors and spike in neurotransmitter activity creates the need for more nicotine to be ingested to achieve the same effect due to a newly developed drug tolerance.<ref> [“The Metabolism of Nicotine .” Metabolism, 2020, www.chm.bris.ac.uk/motm/nicotine/E-metabolisme.html.]</ref> As nicotine stimulates the secretion of these neurotransmitters and receptor activity, the human body is trained to associate a pleasurable response with the usage of nicotine. This activation of the brain's reward pathways; coupled with the stimulation of the adrenal glands, helps create its addictive nature as we become more and more inclined to garner that positive physiological response. | Nicotine(C<sub>10</sub>H<sub>14</sub>N<sub>2</sub>)is a naturally occurring true alkaloid molecule found in tobacco plants. An alkaloid is a class of organic nitrogenous compounds in plants that have prominent physiological impacts on humans. These include, but are not limited to, the inhibition and activation of enzymes, a pronounced effect on nucleic acid and protein synthesis, and effects on membrane structure and cytoskeletal structure and nerve transmission and induction.<ref>[McDaniel, College. “Alkaloids.” Alkaloids, 1999, www2.mcdaniel.edu/Biology/botf99/herbnew/alkaloids.htm.]</ref>Nicotine has gained notoriety in public life due to its addictive nature, which is a direct result of its agonistic behavior in the human brain and nervous system. Nicotine acts as a receptor agonist, a chemical that binds to a receptor and elicits a biological response.<br><br/> Nicotine acts as an agonist to Nicotinic acetylcholine receptors, or nAChRs, a class of receptor polypeptides that respond to the neurotransmitter acetylcholine in the human body. Nicotine competitively binds to these receptors, as it mimics the effects of acetylcholine.<ref>[“The Metabolism of Nicotine .” Metabolism, 2020, www.chm.bris.ac.uk/motm/nicotine/E-metabolisme.html.]</ref><ref>[“Nicotine.” Wikipedia, Wikimedia Foundation, 2 Dec. 2020, en.wikipedia.org/wiki/Nicotine.]</ref><ref>[“Nicotinic Acetylcholine Receptors.” Wikipedia, Wikimedia Foundation, 10 July 2017]</ref>These receptors are found in both nervous systems in the human body, as well as muscles and tissues of the human body. They are the primary receptors at the neuromuscular junction in muscles and are the primary site of nerve and muscle communication. These receptors get their name from nicotine, as nicotine selectively binds to these nicotinic receptors rather than the other receptors in the area.<br><br/>Nicotine is a stimulant, a class of substances that increase physiological and nervous activity in the body. Nicotine stimulates the central nervous system and in turn causes the body to release several neurotransmitters, such as dopamine, acetylcholine, serotonin, and norepinephrine.<ref>[“Nicotine.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/Nicotine.]</ref> However, nicotine only causes a brief uptick in neurotransmitter activity and presence; and the constant activation of these receptors and spike in neurotransmitter activity creates the need for more nicotine to be ingested to achieve the same effect due to a newly developed drug tolerance.<ref> [“The Metabolism of Nicotine .” Metabolism, 2020, www.chm.bris.ac.uk/motm/nicotine/E-metabolisme.html.]</ref> As nicotine stimulates the secretion of these neurotransmitters and receptor activity, the human body is trained to associate a pleasurable response with the usage of nicotine. This activation of the brain's reward pathways; coupled with the stimulation of the adrenal glands, helps create its addictive nature as we become more and more inclined to garner that positive physiological response. | ||
[[File:nicotine.png|thumb|300px|right| <b>Figure 1:</b> A singular molecule of Nicotine. Nicotine is a naturally occurring true alkaloid found in tobacco. It consists of ten carbon atoms, fourteen hydrogen atoms, and two nitrogen atoms.]] | [[File:nicotine.png|thumb|300px|right| <b>Figure 1:</b> A singular molecule of Nicotine. Nicotine is a naturally occurring true alkaloid found in tobacco. It consists of ten carbon atoms, fourteen hydrogen atoms, and two nitrogen atoms. | ||
Link:https://www.acs.org/content/acs/en/education/resources/highschool/chemmatters/past-issues/2019-2020/dec-2019/vaping.html]] | |||
Nicotine is most commonly absorbed through the lungs in the form of smoke, or through smokeless forms of absorption in mucous membranes in the mouth such as chewing tobacco. When used for smoking cessation purposes, or nicotine replacement therapy (NRT), it may be absorbed through buffered alkaline pH systems such as nicotine gum that allow for slower nicotine absorption through cell membranes due to a more gradual integration of nicotine into the bloodstream. Another NRT method, nicotine patches, can be absorbed through the skin in nicotine patches. However, the rate of release into the bloodstream is contingent on many factors, such as permeability of the skin, rate of diffusion, and the rate of nicotine absorption in differing transdermal systems. In consumption outside of NRT, nicotine is metabolized and absorbed very quickly in the body. For example, nicotine absorbed while smoking often reaches the brain in 10 to 20 seconds after the initial puff of a cigarette.<ref>[Benowitz, Neal L, et al. “Nicotine Chemistry, Metabolism, Kinetics and Biomarkers.” Handbook of Experimental Pharmacology, U.S. National Library of Medicine, 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2953858/.]</ref><ref>[Benowitz NL. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. NIDA Res Monogr. 1990;99:12–29.]</ref><ref>[Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743–750.]</ref> | Nicotine is most commonly absorbed through the lungs in the form of smoke, or through smokeless forms of absorption in mucous membranes in the mouth such as chewing tobacco. When used for smoking cessation purposes, or nicotine replacement therapy (NRT), it may be absorbed through buffered alkaline pH systems such as nicotine gum that allow for slower nicotine absorption through cell membranes due to a more gradual integration of nicotine into the bloodstream. Another NRT method, nicotine patches, can be absorbed through the skin in nicotine patches. However, the rate of release into the bloodstream is contingent on many factors, such as permeability of the skin, rate of diffusion, and the rate of nicotine absorption in differing transdermal systems. In consumption outside of NRT, nicotine is metabolized and absorbed very quickly in the body. For example, nicotine absorbed while smoking often reaches the brain in 10 to 20 seconds after the initial puff of a cigarette.<ref>[Benowitz, Neal L, et al. “Nicotine Chemistry, Metabolism, Kinetics and Biomarkers.” Handbook of Experimental Pharmacology, U.S. National Library of Medicine, 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2953858/.]</ref><ref>[Benowitz NL. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. NIDA Res Monogr. 1990;99:12–29.]</ref><ref>[Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743–750.]</ref> | ||
Revision as of 23:11, 8 December 2020
An Introduction to Nicotine
Nicotine(C10H14N2)is a naturally occurring true alkaloid molecule found in tobacco plants. An alkaloid is a class of organic nitrogenous compounds in plants that have prominent physiological impacts on humans. These include, but are not limited to, the inhibition and activation of enzymes, a pronounced effect on nucleic acid and protein synthesis, and effects on membrane structure and cytoskeletal structure and nerve transmission and induction.[1]Nicotine has gained notoriety in public life due to its addictive nature, which is a direct result of its agonistic behavior in the human brain and nervous system. Nicotine acts as a receptor agonist, a chemical that binds to a receptor and elicits a biological response.
Nicotine acts as an agonist to Nicotinic acetylcholine receptors, or nAChRs, a class of receptor polypeptides that respond to the neurotransmitter acetylcholine in the human body. Nicotine competitively binds to these receptors, as it mimics the effects of acetylcholine.[2][3][4]These receptors are found in both nervous systems in the human body, as well as muscles and tissues of the human body. They are the primary receptors at the neuromuscular junction in muscles and are the primary site of nerve and muscle communication. These receptors get their name from nicotine, as nicotine selectively binds to these nicotinic receptors rather than the other receptors in the area.
Nicotine is a stimulant, a class of substances that increase physiological and nervous activity in the body. Nicotine stimulates the central nervous system and in turn causes the body to release several neurotransmitters, such as dopamine, acetylcholine, serotonin, and norepinephrine.[5] However, nicotine only causes a brief uptick in neurotransmitter activity and presence; and the constant activation of these receptors and spike in neurotransmitter activity creates the need for more nicotine to be ingested to achieve the same effect due to a newly developed drug tolerance.[6] As nicotine stimulates the secretion of these neurotransmitters and receptor activity, the human body is trained to associate a pleasurable response with the usage of nicotine. This activation of the brain's reward pathways; coupled with the stimulation of the adrenal glands, helps create its addictive nature as we become more and more inclined to garner that positive physiological response.
Nicotine is most commonly absorbed through the lungs in the form of smoke, or through smokeless forms of absorption in mucous membranes in the mouth such as chewing tobacco. When used for smoking cessation purposes, or nicotine replacement therapy (NRT), it may be absorbed through buffered alkaline pH systems such as nicotine gum that allow for slower nicotine absorption through cell membranes due to a more gradual integration of nicotine into the bloodstream. Another NRT method, nicotine patches, can be absorbed through the skin in nicotine patches. However, the rate of release into the bloodstream is contingent on many factors, such as permeability of the skin, rate of diffusion, and the rate of nicotine absorption in differing transdermal systems. In consumption outside of NRT, nicotine is metabolized and absorbed very quickly in the body. For example, nicotine absorbed while smoking often reaches the brain in 10 to 20 seconds after the initial puff of a cigarette.[7][8][9]
This rapid absorption in turn creates behavioral reinforcement that is quick and easy to create, and this rapidity allows the user to modify the amount of nicotine absorbed, as well as the corresponding effects with the raised level of absorption. This method of modification makes smoking the most dependent form of nicotine addiction, as building tolerance coupled with the modification of intake rapidly accelerates the amount of nicotine needed to garner positive reinforcement. Nicotine absorption is largely dependent on pH. Nicotine has a pKa value of 8.0, making it a weak base. When nicotine is ionized, nicotine crosses membranes much slower than when in its unionized form. Therefore, depending on the method of nicotine consumption, the rate of absorption can be significantly faster or slower. For example, nicotine found in many standard American cigarettes is coupled with an acidic, flue-cured cigarette smoke (pH 5.5-6.0), which generates an ionized form of nicotine that will be slowly and minimally absorbed through the mouth, even if held there for a prolonged period.[10][11][12]
In comparison, nicotine coupled with air-cured tobacco smoke, the tobacco found in cigars and some European cigarettes is more basic (6.5 pH or higher), generating a considerable presence of unionized nicotine. Because the nicotine in this type of smoke is generally in its unionized state, it is more readily absorbed through the mouth, negating the necessity for nicotine exposure to the respiratory system for proper absorption. Due to the minimal absorption through the mouth when smoking standard American cigarettes, the integration of nicotine into the respiratory system is necessary for absorption. The aerial pathways found in the lungs and alveoli provide a large surface area and tight airways allowing for nicotine concentration to be evenly distributed throughout the respiratory tract.
Alongside this even distribution, the basic fluid (7.4 pH) in the human lung allows for nicotine to become about 69% ionized and 31% unionized.[13]This larger presence of unionized nicotine molecules promotes a faster transfer of nicotine across cellular membranes, and the distribution of molecules into the bloodstream, which also provides a basic pH of 7.4. This distribution in turn allows nicotine molecules to bind to receptors in sites such as the liver, kidney, spleen, and lung, leading to potentially damaging effects at these sites.[14]As noted above, after absorption into the bloodstream, nicotine binds to nicotinic receptors at these organ sites and other neuromuscular junctions. The activation of these sites sends signals to the brain that promote neurotransmitter emission and the stimulation of the sympathetic nervous system, leading to pleasurable effects coupled with stimulating effects such as higher heart rate and increases alertness.
Nicotine has been a cause of discussion due to its potentially harmful effects on human cells and microbial cells, as well as its adverse physiological effects.[15]Nicotine has been found to potentially help the development of cancerous cells, as it allows for increased cancer cell survival and proliferation due to its stimulation of nAChRs. Through the activation of particular signal transduction pathways, nicotine allows for damaged cells to survive. Nicotine forms arachidonic acid metabolites which can cause a large increase in cell division, which is extremely problematic when the body is faced with rapid cancer cell growth and division. Chewing tobacco can lead to inflammation of the human gums, which leads to an increased risk of endogenous nitrosation, a process capable of generating carcinogenic N-nitroso compounds in humans.[16]The nitrosation of nicotine can lead to the formation of various carcinogens such as N-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). This is especially prevalent in chewing tobacco users as the inflammation of the gums, combined with the increased risk of carcinogen formation due to the presence of nicotine could lead to an increased risk of cancer.[17]
As nicotine is very addictive, a lack of the drug may also lead to withdrawal symptoms such as headaches, anxiety, depression, and severe nausea, to name a few. Nicotine also puts users at increased risk of heart attacks as it has been suggested that it may lead to the hardening of arterial walls.[18][19] There are also many immediate negative effects of nicotine, such as nausea, diarrhea, vomiting, and abdominal pain. Nicotine increased blood pressure and heart rate, which may also lead to increased risk for cardiovascular dysfunction. However, the effects of nicotine may not all be bad. Nicotine has been shown to increase cognitive function and increase alertness, increase relaxation, and is being researched as a possible remedy for several disorders such as Alzheimer’s Disease, Attention Deficit Hyperactivity Disorder, and Parkinson’s Disease.[20]Nicotine’s activation of the brain’s reward and pleasure center is the main culprit as to why nicotine is so addictive, and the main reason why many patients with various mental health disorders have used it to self-medicate. This contradiction of short term positive effects and potential long term negative effects are a large reason why nicotine has become such an interesting chemical for scientists to research.
Other examples:
Bold
Italic
Subscript: C10H14N2
Superscript: Fe3+
The Effects of Nicotine on Microbial Cells
The effects of nicotine on human cells has been examined in depth, however the effects of nicotine and cigarette smoke are a less studied field that may have large scale implications. One example of this relationship was studied in patients with CRS (chronic rhinosinusitis), a broad spectrum of inflammatory and infectious processes that primarily impact the nose and paranasal sinuses. [21]In many illnesses, a large presence of bacteria is the cause for a prolonged sickness. It has been suggested that there may be a correlation between nicotine, tobacco smoke, and bacterial biofilm formation and virulence.[22]
A bacterial biofilm is defined as a complex, unified, community of bacteria that attach to abiotic and biotic surfaces.[23] The formation of these biofilms allow for microbes to evade host defenses, increase antibiotic resistance, and deploy individual bacteria in order to create other colonies in other areas within the host.[24]
In addition to a plethora of other risk factors, exposure to tobacco smoke has long been touted as a risk factor for the development of CRS.[25]It has been noted that in bacterial samples extracted from CRS patients who use tobacco, the prevalence of biofilm formation is significantly higher than in samples of patients who do not use tobacco. [26]
Bacterial cells that were exposed to tobacco smoke at first, removed from the smoke, and then allowed to grow without the presence of tobacco smoke showed a tendency to revert back to a non biofilm phenotype. [27]This suggests that when infected with an illness in the sinus, continued exposure to tobacco smoke while infected allows bacteria to garner more antibiotic resistance, become more virulent, and continue their lifespan and infection of the host much longer than when tobacco smoke is not present. Bacterial exposure to cigarette smoke and nicotine alters the bacterial life cycle, and in turn makes the presence of these bacteria more problematic. This notion can be applied not only in sinus infections, but in microbial infections of other areas of the body that smoke reaches, including lung infections such as pneumonia and bronchitis. It has been suggested that many illnesses smokers face are refractory, which can be directly attributed to the fact that bacteria are less likely to form biofilms when smoking secedes, and making them easier to treat.
Outside of harmful bacteria, nicotine has been found to provide a stable source of energy for some microbial groups. These microbes, known as “Nicotine-degrading-microorganisms” or “NDMs”, possess the ability to use the carbon and nitrogen atoms found in nicotine molecules as their sole source of the nitrogen and carbon needed for growth.[28] [29][30]These microbes thrive in tobacco rich soil, leaves from the tobacco plant, and in waste formed after the cultivation and use of tobacco. These microbes have been researched and used to improve the quality of cigarettes, dispose of tobacco waste properly, and help to create intermediates of nicotine. Tobacco and nicotine waste has been shown to promote several adverse health effects in humans, so proper removal of this waste is essential in promoting good public health. These microbes have been shown to help reduce the amount of nicotine in tobacco leaves, while still maintaining the same taste of tobacco and assuring the leaf can still be smoked properly.[31][32]
This reduction of nicotine in leaves through microbes helps to detoxify the waste produced from tobacco, and limit the amount of harmful nicotine found in the water and land of areas where waste is deposited. These findings have helped to increase the efficacy of wastewater treatment, and have since generated ways to degrade nicotine faster before it can become a serious health concern.[33][34][35]
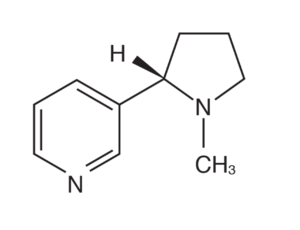
Although bacteria degrade nicotine in various ways, one example of this degradation is A.nicotinovorans, a bacterium that uses the pyridine pathway to attack the 6’ carbon in nicotine through hydroxylation. [36] After this process, various chemical groups such as hydroxyl are introduced, forcing the molecule to restructure and form a new molecular structure. This process allows nicotine to be broken down into smaller components such as formaldehyde and methylamine that are better suited for integration into the environment. Thus, the bacterium is able to utilize the carbon in nicotine for growth, concurrently making the molecule less harmful to the environment.
Another example is Pseudomonas plecoglossicida, a bacteria that has been found to grow in an environment containing high concentrations of nicotine. In this particular case, Pseudomonas plecoglossicida has been found to not only enhance its growth in high concentrations of nicotine, but simultaneously speed up the process of nicotine degradation.This evidence further supports the notion that some microbes not only survive in mediums with high nicotine content, but utilize the molecule to maximize their cellular development.[37]This species of bacteria utilizes the pyrroline pathway of nicotine degradation, a pathway that differs from the conventional metabolic pathways of nicotine degradation.[38]After genomic analysis, researchers have also found this particular species possess three key genes involved in the catabolism of nicotine, spmA, spmB, and spmC. The finding of these genes may also point to the fact that other bacterium with similar genomes could also possess this same metabolic pathway previously unknown to researchers.[39]
The finding that some bacteria metabolize and catabolize nicotine for energy unconventionally is a discovery that could be used in attempts to find the most efficient and environmentally conscious method of waste detoxification, and sheds more light on the diversity of nicotine catabolic pathways in the world around us.
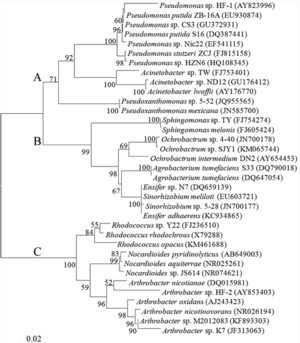
It may also be noted that nicotine, when delivered independently, may have a positive effect on microbial ciliary function. Cilia are small, hair-like protrusions found along the outside of eukaryotic and prokaryotic cells [40]The primary function of cilia in prokaryotes is locomotion, as well as assistance in feeding. It has been found that when Tetrahymena cells were exposed to different smoke extracts, there were both negative and positive impacts on the cell's ciliary function. When exposed to smoke from a traditional cigarette, the rate of vacuole formation in Tetrahymena was nearly four times lower than in cells not exposed to any smoke extract. This evidence suggests that exposure to tobacco smoke may hinder microbial ciliary function. However, when cells were exposed to E-cigarette smoke, a smoke extract with significantly less additives than traditional cigarettes, the rate of vacuole formation increased by 16.25% from the control group’s rate. This suggests that when presented with a medium containing nicotine and minimal additives, cell ciliary action may increase slightly, potentially due to the stimulating effects of nicotine. These findings point to the fact that in traditional cigarettes, it may not be the nicotine that is hindering the ciliary action, rather it is the other chemicals in cigarettes that have a pronounced negative effect on ciliary function in tetrahymena. However, it should also be noted that this experiment only studied the effects of various smoke extracts on Tetrahymena, suggesting that eukaryotic animal cells and other prokaryotes may respond differently when exposed to the smoke extracts used in this experiment.
Section 2 Effects of Nicotine on Animal Cells
Include some current research, with a second image.
The effects of nicotine on human cells has been extensively researched, due to the well known correlation between smoking, nicotine, and various forms of cancer. However, there are other impacts on animal cells that exposure to nicotine presents. Tobacco smoking has long been touted as a risk factor for osteoporosis,
[41]
but the effects of nicotine as an osteoporosis risk factor in humans is still being researched by scientists. Osteoporosis is an imbalance of the growth and resorption of bone, which is dependent on the interactions between osteoblasts and osteoclasts.
[42]
Osteoblasts are bone forming cells that go through a complex process to develop the formation of a mineralized extracellular matrix. It has been found that nicotine has a direct regulatory effect on the development and differentiation of cultured osteoblast-like cells taken from chick embryos [43]
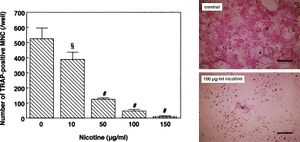
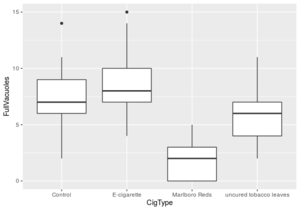
This proposed regulatory effect begs the question of how osteoblast metabolism is affected by nicotine, which researchers have begun to study. To understand the effect of nicotine on osteoporosis risk, researchers concurrently studied nicotine’s effects on osteoclasts, a group of multinucleated cells heavily involved in the resorption of bone. It has been suggested by the European Journal of Pharmacology that “Nicotine may decrease the development of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells and the bone resorption activity of osteoclast-like cells.” [44]
These two findings highlight the notion that nicotine has a positive effect on the differentiation and mineralization of osteoblastic cells, while it has a negative effect on the activation and differentiation of osteoblastic cells. These findings may provide evidence that nicotine itself does not put humans at greater risk of osteoporosis, but the suggestion that nicotine coupled with other compounds in chemicals found in tobacco smoke puts an individual at higher risk for osteoporosis still holds true.
The negative effects of tobacco smoke on human lung epithelial cells have been extensively studied, and with these findings the role of nicotine in the development of lung cancer has become a key point of research. The nitrosation of nicotine can lead to the formation of various carcinogens such as N-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). This is especially prevalent in chewing tobacco users as the inflammation of the gums, combined with the increased risk of carcinogen formation due to the presence of nicotine could lead to an increased risk of cancer. [45]
In epithelial cells it has been found that nicotine, along with the presence of NNK, induces phosphorylation of Akt, a protein kinase that plays a large role in the regulation of cell apoptosis. When Akt is activated, it inhibits apoptosis in multiple tissues in the human body in response to lack of a growth factor or the activation of oncogenes, genes that have the potential to turn a cell into a tumor cell. [46]
Nicotine has been shown to stimulate the phosphorylation of key residues in the gene BCL2 that codes for anti apoptotic protein kinases. [47] However, evidence has emerged that nicotine may “suppress the growth inhibitory effects of trans-retinoic acid (RA) on lung cancer cells by inhibiting retinoic acid receptor (RAR) β expression by inducing expression of an orphan RA receptor TR3, while inhibition of lung cancer cells by retinoid X receptor–selective (RXR-selective) retinoids was unaffected by nicotine” [48]
These findings when coupled together suggest that when acting through specific nAchRs expressed in lung epithelial cells, nicotine, as well as compounds such as NNK that can be formed by exposure to nicotine, may help to expedite the pathogenesis of lung cancer in humans. Nicotine's role in the development of resistance to apoptosis via Akt activation and contact inhibition frames nicotine and other carcinogenic byproducts of nicotine as tumor promoters that may promote the continued growth of genetically damaged cells in the human lung.
As well as having roles in lung cancer development and increased risk for osteoporosis, nicotine has been touted as a molecule that can potentially suppress the human immune system. To supplement this claim, researchers have found that in animals treated with large doses of nicotine showed a significant loss in antibody responses and T-cell proliferation [49]
Nicotine has been shown to have a negative effect on dendritic cell function, suggesting that nicotine interrupts the chain of communication in the immune system by lowering the function of these antigen presenting cells. No evidence has shown that nicotine alters dendritic cell maturation, but their ability to secrete pro-inflammatory cytokines was also negatively affected by the presence of nicotine. [50]
Dendritic cells were noted to have a lesser ability to stimulate T cells when nicotine was present, which is the driving factor behind the reduced rate of T cell proliferation. T cells exposed to nicotine presented regular rates of proliferation. This suggests that nicotine's antiproliferative action on T cells is dependent on its negative effects on dendritic cells and other APC’s. The amount of signal that T cells receive from dendritic cells is a result of the dendritic cells ability to capture, process, and present antigens to T cells, as well as cytokine production. [51]
Without a proper number of T cells, pathogens are able to more readily grow and replicate within a host. It can be noted that nicotine consumption may have an immunosuppressive effect on dendritic cell function, which would in turn make a host more susceptible to infection and disease, as they would lack the T cells needed to provide an appropriate cellular response to foreign pathogens.
Conclusion
Overall text length should be at least 1,000 words (before counting references), with at least 2 images. Include at least 5 references under Reference section.
References
- ↑ [McDaniel, College. “Alkaloids.” Alkaloids, 1999, www2.mcdaniel.edu/Biology/botf99/herbnew/alkaloids.htm.]
- ↑ [“The Metabolism of Nicotine .” Metabolism, 2020, www.chm.bris.ac.uk/motm/nicotine/E-metabolisme.html.]
- ↑ [“Nicotine.” Wikipedia, Wikimedia Foundation, 2 Dec. 2020, en.wikipedia.org/wiki/Nicotine.]
- ↑ [“Nicotinic Acetylcholine Receptors.” Wikipedia, Wikimedia Foundation, 10 July 2017]
- ↑ [“Nicotine.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/Nicotine.]
- ↑ [“The Metabolism of Nicotine .” Metabolism, 2020, www.chm.bris.ac.uk/motm/nicotine/E-metabolisme.html.]
- ↑ [Benowitz, Neal L, et al. “Nicotine Chemistry, Metabolism, Kinetics and Biomarkers.” Handbook of Experimental Pharmacology, U.S. National Library of Medicine, 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2953858/.]
- ↑ [Benowitz NL. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. NIDA Res Monogr. 1990;99:12–29.]
- ↑ [Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743–750.]
- ↑ [Gori GB, Benowitz NL, Lynch CJ. Mouth versus deep airways absorption of nicotine in cigarette smokers. Pharmacol Biochem Behav. 1986;25(6):1181–1184.]
- ↑ [Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol. 2001;14(11):1465–1481.]
- ↑ [Benowitz, Neal L, et al. “Nicotine Chemistry, Metabolism, Kinetics and Biomarkers.” Handbook of Experimental Pharmacology, U.S. National Library of Medicine, 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2953858/.]
- ↑ [Benowitz NL, Jacob P, 3rd, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982a;221(2):368–372]
- ↑ [Benowitz, Neal L, et al. “Nicotine Chemistry, Metabolism, Kinetics and Biomarkers.” Handbook of Experimental Pharmacology, U.S. National Library of Medicine, 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2953858/.]
- ↑ [Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–9.]
- ↑ [Hoffmann D, Adams JD. Carcinogenic tobacco-specific N-nitrosamines in snuff and the saliva of snuff dippers. Cancer Res. 1981;41(11 Pt 1):4305–8.]
- ↑ [Hoffmann D, Adams JD. Carcinogenic tobacco-specific N-nitrosamines in snuff and the saliva of snuff dippers. Cancer Res. 1981;41(11 Pt 1):4305–8.]
- ↑ [Unknown. “How Smoking and Nicotine Damage Your Body.” Www.heart.org, www.heart.org/en/healthy-living/healthy-lifestyle/quit-smoking-tobacco/how-smoking-and-nicotine-damage-your-body.]
- ↑ [McLaughlin, Ian, et al. “Nicotine Withdrawal.” Current Topics in Behavioral Neurosciences, U.S. National Library of Medicine, 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4542051/.]
- ↑ [Publishing, Harvard Health. “Nicotine: It May Have a Good Side.” Harvard Health, www.health.harvard.edu/newsletter_article/Nicotine_It_may_have_a_good_side.]
- ↑ [Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117:S1–7.]
- ↑ [Ramadan HH, Sanclement JA, Thomas JG. Chronic rhinosinusitis and biofilms. Otolaryngol Head Neck Surg. 2005;132:414–417.]
- ↑ [Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW, et al. Microbial interactions and differential protein expression in Staphylococcus aureus -Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59:493–503.]
- ↑ [Palmer JN. Bacterial biofilms: do they play a role in chronic sinusitis? Otolaryngol Clin North Am. 2005;38:1193–1201, viii.]
- ↑ [US Department of Health EaW, US Public Health Service. Washington DC: 1964. Smoking and Health: Report of the Advisory Committe to the Surgeon General of teh Public Health Service.]
- ↑ [Goldstein-Daruech, Natalia, et al. “Tobacco Smoke Mediated Induction of Sinonasal Microbial Biofilms.” PloS One, Public Library of Science, 6 Jan. 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3017060/.]
- ↑ [Goldstein-Daruech, Natalia, et al. “Tobacco Smoke Mediated Induction of Sinonasal Microbial Biofilms.” PloS One, Public Library of Science, 6 Jan. 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3017060/.]
- ↑ [Brandsch R (2006) Microbiology and biochemistry of nicotine degradation. Appl Microbiol Biotechnol 69:493–498,]
- ↑ [Li HJ, Li XM, Duan YQ, Zhang KQ, Yang JK (2010) Biotransformation of nicotine by microorganism: the case of Pseudomonas spp. Appl Microbiol Biotechnol 86:11–17,]
- ↑ [Gurusamy R, Natarajan S (2013) Current status on biochemistry and molecular biology of microbial degradation of nicotine. Sci World J 125385]
- ↑ [Chen CM, Li XM, Yang JK, Gong XW, Li X, Zhang KQ (2008) Isolation of nicotine-degrading bacterium Pseudomonas sp. Nic22, and its potential application in tobacco processing. Int Biodeterior Biodegrad 62:226–23]
- ↑ [Li HJ, Li XM, Duan YQ, Zhang KQ, Yang JK (2010) Biotransformation of nicotine by microorganism: the case of Pseudomonas spp. Appl Microbiol Biotechnol 86:11–17]
- ↑ [Wang MZ, Zheng X, He HZ, Shen DS, Feng HJ (2012a) Ecological roles and release patterns of acylated homoserine lactones in Pseudomonas sp. HF-1 and their implications in bacterial bioaugmentation. Bioresour Technol 125:119–126]
- ↑ [Wang HJ, He HZ, Wang MZ, Wang S, Zhang J, Wei W, Xu HX, Lv ZM, Shen DS (2013a) Bioaugmentation of activated sludge with Acinetobacter sp. TW enhances nicotine degradation in a synthetic tobacco wastewater treatment system. Bioresour Technol 142:445–453]
- ↑ [Wang X, Tang L, Yao YL, Wang HX, Min H, Lv ZM (2013b) Bioremediation of the tobacco waste-contaminated soil by Pseudomonas sp. HF-1: nicotine degradation and microbial community analysis. Appl Microbiol Biotechnol 97:6077–6088.]
- ↑ [G. Akcay, K. Yurdakoc, et al. “Nicotine-Degrading Microorganisms and Their Potential Applications.” Applied Microbiology and Biotechnology, Springer Berlin Heidelberg, 1 Jan. 1970, link.springer.com/article/10.1007/s00253-015-6525-1.]
- ↑ [Raman G, Mohan KN, Manohar V, Sakthivel N (2014) Biodegradation of nicotine by a novel nicotine-degrading bacterium, Pseudomonas plecoglossicida TND35 and its new biotransformation intermediates. Biodegradation 25:95–107]
- ↑ [Gurusamy R, Natarajan S (2013) Current status on biochemistry and molecular biology of microbial degradation of nicotine. Sci World J 125385]
- ↑ [Tang H, Wang L, Wang W, Yu H, Zhang K, Yao Y, Xu P (2013) Systematic unraveling of the unsolved pathway of nicotine degradation in Pseudomonas. PLoS Genet 9:e1003923]
- ↑ [Editors, BD. “Cilium.” Biology Dictionary, 25 June 2017, biologydictionary.net/cilium/.]
- ↑ [P.D. Broulik, J. Jarab The effect of chronic nicotine administration on bone mineral content in mice Horm. Metab. Res., 25 (1993), pp. 219-2210.]
- ↑ [B.L. Riggs Pathogenesis of osteoporosis Am. J. Obstet. Gynecol., 156 (1987), pp. 1342-1346]
- ↑ [W.K. Ramp, L.G. Lenz, R.J.S. Galvin Nicotine inhibits collagen synthesis and alkaline phosphatase activity, but stimulates DNA synthesis in osteoblast-like cells Proc. Soc. Exp. Biol. Med., 197 (1991), pp. 36-43]
- ↑ [Yuhara, Sachiko, et al. “Effects of Nicotine on Cultured Cells Suggest That It Can Influence the Formation and Resorption of Bone.” European Journal of Pharmacology, Elsevier, 9 Dec. 1999, www.sciencedirect.com/science/article/pii/S0014299999005518?casa_token=2oebpy_oF8kAAAAA%3A7aiZ8IVqfo-V_xWYDZJwe6gXqN0Iy63a5YCJvaAhTFjJrrYiPHt4sG-GLuVHGI-BETGaJ_E_WXw.]
- ↑ [Hoffmann D, Adams JD. Carcinogenic tobacco-specific N-nitrosamines in snuff and in the saliva of snuff dippers. Cancer Res. 1981;41(11 Pt 1):4305–8.]
- ↑ [Minna, John D. “Nicotine Exposure and Bronchial Epithelial Cell Nicotinic Acetylcholine Receptor Expression in the Pathogenesis of Lung Cancer.” The Journal of Clinical Investigation, American Society for Clinical Investigation, 1 Jan. 2003, www.jci.org/articles/view/17492.]
- ↑ [Mai, H., May, W.S., Gao, F., Jin, Z., and Deng, X. 2002. A functional role for nicotine in Bcl2 phosphorylation and suppression of apoptosis. J. Biol. Chem. doi:10.1074/jbc.M209044200.]
- ↑ [Chen, GQ, Lin, B, Dawson, MI, Zhang, XK. Nicotine modulates the effects of retinoids on growth inhibition and RAR beta expression in lung cancer cells. Int. J. Cancer. 2002. 171-178.]
- ↑ [Geng Y, Savage SM, Razani‐Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol 1996; 156 : 2384–90.]
- ↑ [Nouri‐Shirazi, Mahyar, and Elisabeth Guinet. “Evidence for the Immunosuppressive Role of Nicotine on Human Dendritic Cell Functions.” Wiley Online Library, John Wiley & Sons, Ltd, 16 June 2003, onlinelibrary.wiley.com/doi/full/10.1046/j.1365-2567.2003.01655.x.]
- ↑ [Nouri‐Shirazi, Mahyar, and Elisabeth Guinet. “Evidence for the Immunosuppressive Role of Nicotine on Human Dendritic Cell Functions.” Wiley Online Library, John Wiley & Sons, Ltd, 16 June 2003, onlinelibrary.wiley.com/doi/full/10.1046/j.1365-2567.2003.01655.x.]
Edited by Tyler Dougherty, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2020, Kenyon College.
