Effects of Pathogen-Vector Interactions on the Transmission of Dengue Virus
Effects of Pathogen-Vector Interactions on the Transmission of Dengue Virus
A Viral Biorealm page on the family Effects of Pathogen-Vector Interactions on the Transmission of Dengue Virus
Dengue Virus
Group IV: ss(+)RNA virus
Family: Flaviviridae
Genus: Flavivirus
Dengue virus (DENV) is the causative agent of both classical dengue fever, and the more severe manifestations; dengue hemorrhage fever (DHF) and dengue shock syndrome (DSS)[4]. It is most commonly transmitted by the mosquito vector Aedes aegypti as seen in figure 2, but can be transmitted by other members of the genus Aedes including Aedes albopictus[5]. There are four different serotypes of dengue virus (DENV 1-4). Infection with one serotype affords life-long immunity to that serotype but only partial (heterologous) immunity to other serotypes for a short period of time post-infection. After initial protective immunity the risk of developing DHF or DSS upon reinfection strain from a different serotype increases.
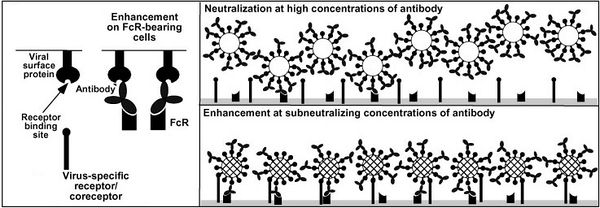
This increased risk for severe dengue is the result of antibody-dependent enhancement of viral infection. Host heterotypic non-neutralizing antibodies from previous DENV infection bind DENV virions. After recent DENV infection there are enough antibodies to neutralize any new dengue viruses but overtime the number of neutralizing antibodies drops. At some point there are so few enough antibodies left that upon reinfection with a new strain the anitbodies can bind but not nuetralize the virus. The constant region of the antibody can go on to bind a FcγR receptor on the surface of a number of different types of immune cells.Normally, when a pathogen-antibody complex binds to a FcγR receptor a cytoxic or phagocytoic response initiated by the immunne cell. DENV somehow avoids this response and instead binds its host cell surface receptor with unbound E protiens and infects the cell as seen in figure 3.
The FcγR receptors are effect in controlling other pathogens but DENV has evolved to exploit this pathway. The FcγR receptor is not needed for DENV entry but does increase infectivity DENV and allows the virus display cell tropisms not seen in primary DENV infection. With more cells infected more viroins are produced leading to higher levels of viremia in the blood which is correlated with increased risk for DHF and DSS.
increase infectivity of the virus in immune cells by increasing overall viral load within the patient. Higher levels of viremia are associated with incresed the risk of DHF and DSS [3,?]
Within serotypes dengue viruses are organized by genotypes, subtypes, clades, variants, groups and finally strains [3]. The large number of dengue strains in the world combined with only partial crossover immunity to other strains makes concurrent (multi-strain) infections or reoccurring dengue infections possible (just like repeated bouts of the flu), especially in areas with high prevalence of DENV.
Dengue viruses are now endemic in many tropical parts of the world putting about a third of the world’s population at risk of infection. There are estimated to be over one hundred million new infections per year and rising [5,6]. Rising rates of infection are the result of reduced multiple factors that affect vector population and range. vector control in areas that need it most has been cutback and global warming has expanded vector ranges to areas previously DENV free. Milder winters at higher latitudes are allowing adult and larvae mosquitoes to survive longer into the fall and return earlier in the spring allowing the DENV transmission cycle to continue throughout a greater part of the year than in the past. DENV is becoming a threat to industrialized nations once thought to be too far away from the tropics for Dengue fever to be a threat. In the U.S. the number of indigenous dengue cases is rising. In Brownsville Texas, 25% of the residents who had never traveled outside the united states had antibodies indicating prior exposure to a strain of DENV that had to have maintained fairly silent transmission for quite some time [6,7].
The severity of dengue infection also continues to increase. The ratio of dengue DHF and DSS cases to classical dengue fever have increased dramatically over the past sixty years. It has become the leading cause of hospitalization and death in children in several endemic countries [7].
Dengue Shock Syndrome and Dengue Hemorrhagic Fever
Although classical dengue fever is not usually fatal it has very high morbidity; its alternate name is break-bone fever because of the severe joint pain during infection. DENV infection is most common in infants, young children, and adults. Classical dengue fever manifests as a mild to high fever with red rash, debilitating headaches, muscle and joint pain.
The more severe manifestations of the disease are dengue hemorrhage fever and dengue shock syndrome. Young children and those with antibodies from previous DENV infection are most at risk for these complications because of antibody-dependent enhancement of infection[5].
Dengue shock syndrome results from DENV infection of the endothelial cells lining the blood vessel [17]. When these cells become damaged the integrity of the blood vessels is compromised and plasma leaks out, causing a host of systemic problems including edema and hypotenstion. The swelling of tissue because plasma escaping the circulatory system (edema) causes abdominal pain. Hypotension is a drop in blood pressure that results from a drop in total blood volume from plasma loss. Edema and hypotension both impede proper circulaton.Oxygen and nutrients stop reaching the body's tissues possibly leading to shock and death [6].
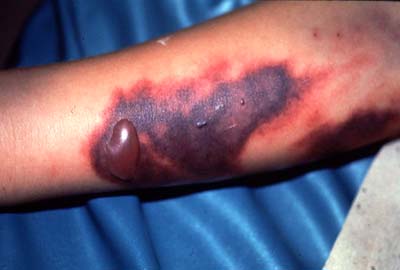
Dengue hemorrhage fever occurs when normal blood coagulation is disrupted by infection. Fever, emesis (vomiting), and hemorrhaging (bleeding) are all symptoms common to this type of dengue infection[5]. The results of internal hemorrhaging are visible on the skin in the form of tiny red spots (petechiae) or sometimes patches under the skin (ecchymosis)as seen in figure 4. As well as bloody stool (feces) and bleeding from the gums and nose (Figure 3)[5]. Mortality from these complications can be up to 14% without proper care [4].
Unfortunately there are no anti-viral drugs for DENV infection. The only treatment is supportive care in the form of fluid replacement and pain management [5]. With no licensed vaccine, disruption of the vector-host transmission cycle is the only form of disease control. To stop transmission from host to vector, those infected only need to prevent further mosquito bites which can easily be achieved with the use of mosquito nets as shown in figure ?.
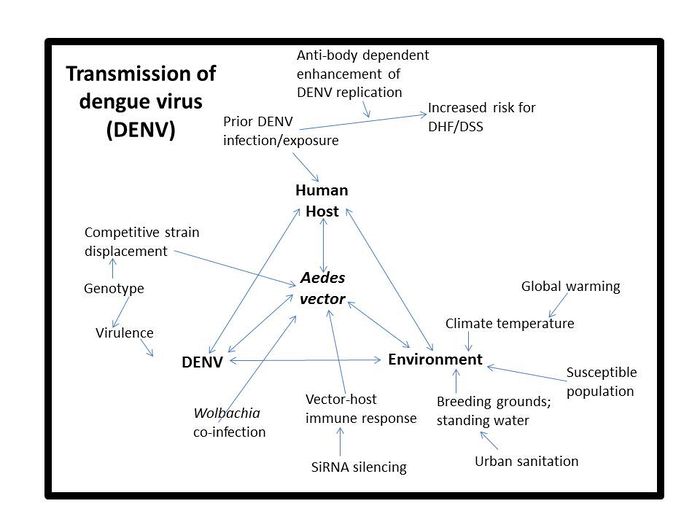
Controlling the transmission of dengue virus is simple but labor-intensive and costly. In the past heavy pesticides such as DDT were used to wipe out mosquito populations, but this has fallen out of favor as the environmental toxicity of pesticides has come to light [7]. Nowadays, active disease control centers around public health campaigns that urge citizens to get rid of standing water in and around their homes and seek treatment if they have dengue fever-like symptoms. Use of insect repellents to is also encouraged as to reduce risk of mosquito bite, as inferred in figure 5. Current DENV education campaigns and vector control programs are not as cost-effective as other public programs and this has led to a decrease in government funding over the years in areas that need it most. In an attempt to make vector control programs more efficient, researchers are trying to understand the complexities of dengue virus replication in mosquito vectors. Coinfection of the mosquito vector with multiple DENV strains or the bacterium Wolbachia and interactions between DENV and the vector’s immune system all modulate viral replication within the vector. The replication kinetics of DENV in the vector-host have because of its overreaching effects on the epidemiology of the disease [5].With a better understanding of how each of these factors affects the overall viral load and rate of viral replication with in the vector, it will be possible to come up with better strategies for vector transmission control and prevention as well as the mitigation of future DENV epidemics (figure DENV triangle).
Types of DENV strains
There are multiple ways to classifications DENV strains depending on the context. Dengue viruses maintaining transmission mostly between nonhuman primates and forest dwelling Aedes aegypti are considered sylvatic. It was once thought that sylvatic strains were less virulent than epidemic or endemic strains of DENV. Some believed sylvatic strains lacked the potential to cause virulent epidemics because cases of sylvatic dengue fever are rare when compared to overall incidence of of dengue fever. As it turns out, sylvatic strains share the same pathogenic potential as strains found in urban areas. There are reported cases dengue hemorrhagic fever from infection with sylvatic DENV. The destruction of forest areas where sylvatic strains of DENV are maintained has not even lead to a decrease in outbreaks of sylvatic DENV in West Africa [18].
Epidemic and endemic DENV strains infect and replicate within humans and domesticated Aedes species [9].
The distinction between epidemic and endemic dengue has become hazy as epidemic strains of DENV have become endemic in tropical urban slums. In areas where there is poor sanitation, free standing water, high population density, and constant human traffic allow continuous transmission of the virus (Figure 1)[4,8]. Epidemic strains of DENV are best transmitted by Aedes ageypti mosquitoes but can be transmitted less efficiently by other Aedes species and cause[19]. Epidemic strains can also produce large fast moving epidemics if a number of factors create high density vectors populations in close proximity to high density host populations [20].
Invassive species are defined as strains or genotypes of DENV not previously seen in a particular area or geographic region [14]. They tend to cause large epidemics because the population in that area does not have homologous immunity to the virus. Noninvasive species are endemic to the area and maintain slow continued transmission. Invassive species can be more virulent than noninvassive strains but this is dependent on host population susceptibility all of the strains of DENV circulating in the area at that given time. An invasive strain can colonize a given area if it can outcompete other DENV strains for susceptible hosts, eventually becoming and established endemic strain to the area.The differentiation between innvassive and noninvassive is an arbitrary tern to differentiate between established and new species of DENV.
DENV can also be classified according to genotype. The two most common genotypes mentioned in the literature are American and Southeast Asian because of their well-documented differences in virulence. American genotype strains tend to be less virulent as well as less competitive than Southeast Asian genotype strains.
Viral Replication
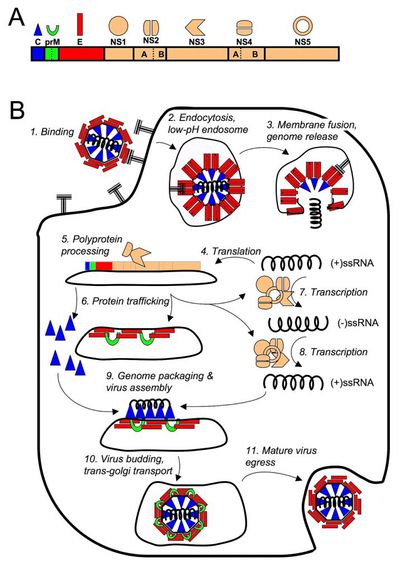
DENV infection begins at the cellular level when E proteins on the surface of a DENV virion binds to a host cell receptor initiating endocytosis. The high cell tropism of DENV has made the elucidation of a single host cell receptor difficult and the exact host cell receptor or receptors responsible for viral entry remain to found [22].
Once endocytosed, DENV escapes the endosome to the cytoplasm where it can immediately begin translation of its genome. Like other (+)ssRNA viruses it must transcribe a negative strand RNA template to transcribe new copies of its genome. Mature DENV capsids are eventually enveloped in host cell membrane and viral E protein when they bud out of the host cell.
Transmission Cycle
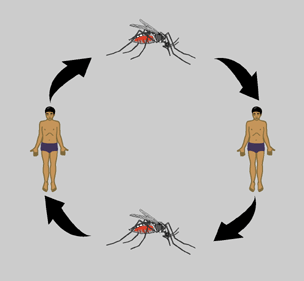
The DENV transmission cycle begins when an infected mosquito bites a human or nonhuman primate host. The Host begins to experience symptoms four to seven days post-infection with symptoms usually lasting three to ten days after which there is about a three week recovery period [8]. The virus initially replicates in target organs like the liver, spleen, and thymus but eventually makes its way to the lymphatic system where it replicates in lymph tissue and white blood cells. The lymphatic system serves as a gateway to the bloodstream where the virus continues to replicate and can potentially infect the endothelial cells of the circulatory vasculature. Eventually virus titers are high enough in the host blood for disease transmission to occur. For a period of about five days, just before symptoms appear, the virus is transmissible to a new mosquito vector through an infected blood meal.
After being taken up in an infected blood meal by a mosquito DENV replicates in the midgut of the insect and infects its hemocoel (body cavity). From the hemocoel, the virus eventually infects the salivary glands. Once virus titers are high enough in the salivary glands the mosquito can infect a new susceptible host when it goes for another blood meal [4]. The length of time required for virus to reach the salivary glands of a mosquito after the ingestion of an infected blood meal is about eight to ten days depending on both viral and host factors [8]. The delay between ingestion of an infected blood meal and infection and replication of DENV in the salivary glands influences the course of epidemics and plays a role in competition between DENV strains.
Viral factors that affect DENV replication and transmission in the mosquito vector
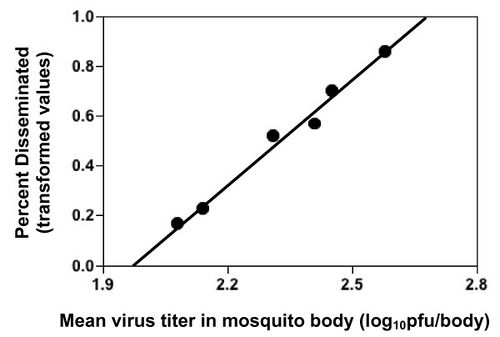
The transmission from vector to host for a particular DENV strain is determined by viral infectivty of mosquito cells, and the rate of viral replication in these cells. Dissemination from the heomocoel to the salivary glands affects disease transmission as well but it is tied directly tied to viral replication rates and viral titers in the hemocoel as seen in figure ?[4]. Replication rate and viral titers are both linked to genotypic differences between DENV strains. Strains belonging to more virulent genotypes have fast rates of viral replication as well as higher virus titers in human and vector hosts[4,23].
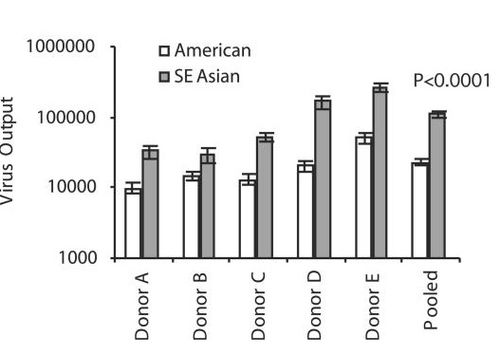
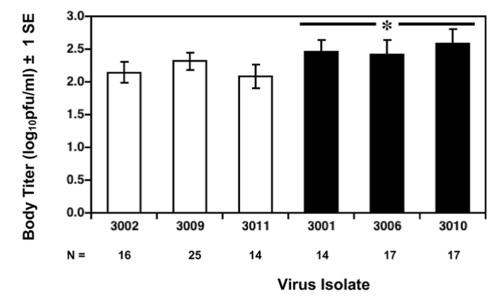
Figure ? shows greater viral output (defined as number of viral genomes over the number of infected cells) in donor dendritic cells 48 hours post-infection. Black bars represent data pooled from tweleve different Southeast Asian genotype strains, white bars represent data pooled from seven different American strains.
In figure ? it is clear that the Invassive DENV-3 strains had higher viral titers than noninvassive strains.
Differences in the encoded secondary structure nontranslated regions of the RNA genome (NTRs) are correlated with differences in virulence between strains. No specific nucleotide or amino acid differences in either the coding or noncoding region have been correlated with attenuated disease in humans. It is only clear that there are differences at these sights between more virulent strains and less virulent strains (figure ?). differences in the three dimmensional structure of the viral genome may affect the initation of translation. This could explain why more virulent strains have higher rates of replication.Mutations in the sequence with in the envelope glycoprotein E have also been correlated with virulence but as of yet there are no specifc changes in nucloetide sequnce correlated with virulence [23].
Vector Host-Factors that Affect DENV Replication and Transmission in Mosquito Vector
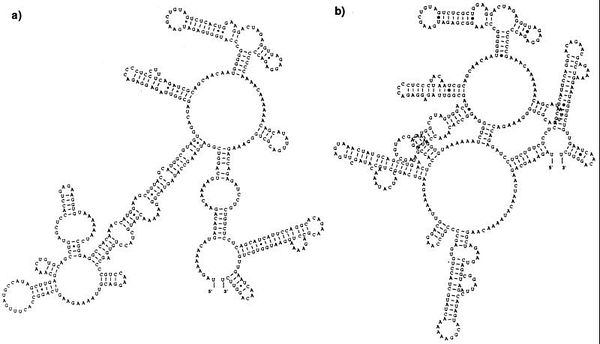
The Aedes vector's innate immune response to DENV infection influences the rate of DENV replication, which influences the lag period between initial infection in the mosquito and disease transmission between mosquito and host (extrinsic incubation period)[4,6]. mean DENV titers in infected mosquitoes can be modulated by blocking the function of one or more components in the RNA interference (RNAi) pathway. This innate immune response is triggered by the presence of dsRNA in an infected cell. In the case of DENV infection the secondary structure of the NTR of the RNA genome may trigger this response. The hairpin loops as seen in figure (the structure one) look like dsRNA[24]. Activation of the RNAi pathway leads to the degradation of dsRNA within the infected cell. Although it does not completely inhibit infection, virus titers are lower in mosquito cells with a functional RNAi response than mosquito cells without a mutant or disabled response [24]. DENV dsRNA can also activate Toll pathways in infected mosquito cells. Active Toll pathways upregulate the production of a variety peptide antimicrobials meant to destroy the virus [25].
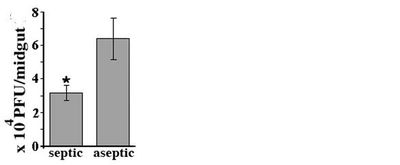
The presence of bacterial flora in the gut of mosquitoes also upregulates the mosquito vector's Toll pathway response, creating an immune response that keeps gut microbiota in check as well as limiting DENV replication. In figure ? the DENV titers in the midgut of infected mosquitoes raised in a microbe free environment (aseptic) are much higher than mosquitoes raised septically [25].
Infection of the bacterium Wolbachia decreases the ability of mosquito vectors to tranmitt DENV by shortening the life-span of the vector. The less time a mature vector with high DENV titers is around potentially biting people the less chance there is for disease transmission. Over-replication of Wolbachia in host cells also seems to be responsible for changes in vector feeding behaviour leading to fewer succesful transmissions from mosquito to host during blood-feeding. Intentional infection of vector populations with wolbachia is currently being considered as a novel biocontrol strategy in vector control [13,26].
Competitive Strain Displacement Between Dengue Strains
There are multiple ways a strain prevalent in a given geographic area can change. A strain can evolve over time until it is antigenetically different enough from the original strain so that infection with the original parent strain does not confer homologous immunity to this daughter strain making it a new strain [4]. Strains can go extinct in a given geographic area because there are no more susceptible human hosts left leaving room for a strain prevelant in given in an area to take over. Or, a new strain can be introduced to an area and outcompete existing strains for susceptible hosts. Competitive strain displacement has been documented in epidemiological data as well controlled studies involving coinfection of multiple strains of dengue in a single host [14].
Competitive strain displacement occurs when a more virulent strain of DENV competes with a less virulent strain, resulting in a shift in the epideimiology of dengue fever cases within a given area that reflects the characteristics of the invading virulent strain. Competition occurs during singular strain infection of a DENV vector or co-infection of a vector with multiple strains of DENV. In singular infection of a vector the more virulent strain can replicate and disseminate faster in the vector host decreasing the extrinsic incubation period. A shorter viral incubation period in the vector increases the number of days a mosquito is infectious and potentially transmitting disease. The longer the mosquto is infectious before it dies the more people can be infected by this strain. The more virulent strain has more chances of being transmitted from vector to host increasing the number of DENV infections due to this particular strain in a given population.
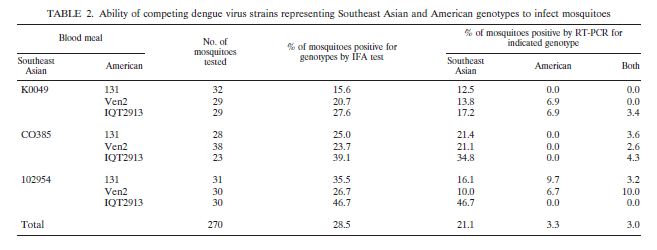
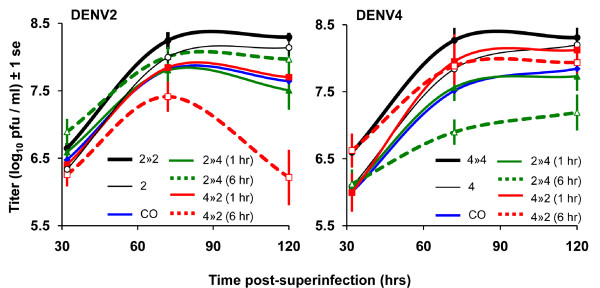
Competitive strain displacement is also seen during co-infection of a vector with or more strains of DENV. The more virulent strain tends to be able to replicate faster increasing the ratio between more virulent virus particles and less virulent virus particles. Figure ? shows the results of vector coinfection between a virulent strains of the Southeast Asian genotype and less virulent strains of the American genotype.
Again, competition is a numbers game. The mosquito vector can transmit both strains of the virus equally but, if there is a higher viral titer of one strain over another, the coinfected vector will be more likely to transmitt the strain with the higher viral titer to a new host.
There are a number of studies comparing strains from each genotype
They are commonly compared because of known differences in virulence genotype which allows researchers to look for differences in replication, infectivty and other factors that may explain why certain strains of DENV are worse than others. . In both mosquitoe and human dendritic cells Southeast Asian genotypes have higher rates of replication than American genotypes resulting in higher viral titers in humans….uhhhh
Southeast Asian genotypes also outcompete American genotypes when coninfecting mosquito hosts by replicating and disseminating to the salivary glands faster.
Competitive strain displacement also occurs on the human side of the viral life cycle but is not as much of a concern when looking at ways to prevent the spread of more virulent dengue strains because of methods of transmission control already discussed.
is able to suppress viral replication of the less virulent strain to become the more frequently transmitted strain [4,13].
It makes sense that the more virulent strain is also more competitive. Virulence in humans is correlated with increased levels of viremia which result from higher infectivity of cells and increased rates of translation of the viral proteome which increases virion replication. Competeitivity in vector hosts is measured by the rate of dissemination of the virus from infected blood meal to the salivary glands of the mosquito and this is also affected by the infectivy of the infecting strain and it’s rate of replication with in cells. What causes more severe disease in humans also translates to increased transmission in vectors.
This phenomenon is one way to explain the increase in the number of severe cases of dengue (dengue hemorrhage fever and dengue shock syndrome) over the past fifty years and the global turnover of dengue strains. The distribution of dengue serotypes in areas with endemic dengue has not changed over the years but the prevalence of different genotypes, subtypes or groups (that have varying levels of virulence in humans) continues to change around the globe. Invasive strains (new to a given geographic area) tend to be more virulent than the native strain already present and therefore are able to become more prevalent than the native strain through competitive displacement [4,13].
In Sri Lanka in the 1980’s an invasive DENV3 strain was introduced that eventually displaced the native DENV3 strain resulting in a dramatic increase in the number of severe dengue cases. Since the 1980’s all four dengue serotypes have been circulating freely in Sri Lanka but only after 1989 was there a sharp rise in cases of dengue hemorrhagic fever and dengue shock syndrome [14].
In both single and co-infection of a vector with dengue viruses there are multiple variables that affect the transmissibility of strain which in turn affects how competitive one strain is relative to another. They include; level of infectivity (how well the virus can infect the vector), rate of dissemination from the midgut to the salivary glands, and replication rate within the midgut and salivary glands. Depending on the strains being compared the infection rate of the vector by an invasive (and usually more virulent strain) is either the same or higher compared to the native strain [4,13]. Faster dissemination rates in single strain infections between invasive and native strains have also been observed. Rate of virus replication within both the midgut and salivary gland are higher in invasive strains as well [4,13].
Vectoral capacity is the frequency at which a mosquito bites and can potentially transmit infection to a human host following ingestion of an infected blood meal. It is determined by a number of factors including length of vector survival and vector competence [13]. Vectoral survival is the length of time the vector (mosquito) lives (and can bite and infect hosts) after infection. Vectoral competence is the capacity of a vector to be infected by, maintain, and transmit a virus. In vectors infected with the more virulent invasive strains vectoral capacity is heightened which increases frequency of transmission and prevalence of the invasive strain. Although the exact cause is unknown, mosquitoes infected with invasive strains are more likely to survive the incubation period of the virus (the first eight to ten days after infection) and go on to transmit dengue virus than mosquitoes infected with native strains. This especially holds true when the overall vector survival rate is low due to environmental factors such as temperature and humidity [13].
In studies of co-infection of vector cells with more virulent and less virulent strains, the more virulent strain was more competitive than the less virulent strain. Co-infection of strains in mosquito cells reduced viral titers of both strains but the level of replication suppression in the more virulent strains was much lower than the level of suppression in the less virulent strains. This effect was most pronounced at high MOIs (initial amount of virus cells were exposed to during infection). High levels of infecting virus increased the competition between strains and there was increased suppression of viral replication seen in the less virulent strain.
Superinfection of the same vector can decrease overall titers of both infecting strains (asymmetric competitive suppression) and if the innvassive strain is less competitive/virulent than existing dengue strains in may not be able to colonize that geographic area (asymmetric as well as selection for dengue)
Epidemiologic Consequences of Various Forms of Vector Control
Even slight changes in the pathogen-vector dynamic at the cellular level can affect dengue virus transmission. Minute changes of virus titers in the salivary glands, rate of dissemination of virus from the midgut to the salivary glands as well as a slew of other changes due to pathogen-vector dynamics can have a far reaching impact on the epidemiology of DENV infections. With a better understanding of the involvement of dengue virus genotype and vector host factors in disease transmission it may be possible to manipulate one of these factors that influence the pathogen-vector dynamic to help prevent further spread of dengue fever even without dengue specific drugs or a vaccine.
References
[1] Cann, A., September 25, 2007. “Dengue virus” Flickr. Available: http://www.flickr.com/photos/ajc1/1437910835/ [Date visited: 12/7/10]
[2] Funchal Camara Municipal. “Monitoring the Yellow Fever Mosquito” Funchal Camara Municipal. Available: http://www.cm-funchal.pt/Cmf/en/Default.aspx?ID=110 [Date visited: 11/1/10]
[3] Takada, A., Y., Kawaoka. 2003. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13: 387-398
[4] Hanley, K.A., J.T., Nelson, E.E., Schirtzinger, S.S., Whitehead, C.T., Hanson. 2008. Superior infectivity for mosquito vectors contributes to competitive displacement among strains of dengue virus. BioMed Central Ecology 8(1)
[5] Centers for Disease Control and Prevention. October 28, 2010. “Dengue” Available: http://www.cdc.gov/dengue [Date visited: 10/28/10]
[6] Dengue Fever. 2010. “Dengue Fever Symptoms” denguefeversymptoms.org. Available: http://www.denguefeversymptoms.org/ [Date visited: 10/31/10]
[7]Marsa, L. 2010. The hot zone. Discover December 2010
[8] World Health Organization. 2010. “Dengue” Available: http://www.who.int/topics/dengue/en/ [Date visited: 10/27/10]
[9] Pepin, K.M., K.A., Hanley. 2008. Density-dependent competitive suppression of sylvatic dengue virus by endemic dengue virus in cultured mosquito cells. Vector Borne Zoonotic Diseases. 8(6): 821-828
[10] Prima Quality Media. 2010. “gdeng052.gif” Available: http://www.primaquality.com/2008/03/30/combating-of-dengue-hemorrhagic-fever-scourge/cyrcle-transmission-of-dengue-virus/ [Date visited: 10/29/10]
[11] Genesio, J.. May 21, 2010. “Beware of the Bite” Natural Unseen Hazards Blog. Available: http://naturalunseenhazards.wordpress.com/2010/05/21/recent-outbreak-of-dengue-cases-are-the-first-locally-acquired-cases-in-florida-since-1934/ [Date visited: 10/30/10]
[12] Worldnews Infectious Disease. 2010. “Recovering dengue fever patient” Available: http://wn.com/infectious_disease [Date visited: 10/28/10]
[16] Moreira, L.A., I., Iturbe-Ormaetxe, J.A., Jeffery, G., Lu, A.T., Pyke, L.M., Hedges, B.C., Rocha, S., Hall-Mendelin, A., Day, M., Riegler, L.E., Hugo, K.N., Johnson, B.H., Kay, E.A., McGraw, A.F., Van Den Hurk, P.A., Ryan, S.L., O’Neill. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and plasmodium. Cell. 139: 1268-1278
[13] Anderson, J.T., R., Rico-Hesse. 2006. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. American Journal of Tropical Medicine and Hygiene. 75(5):886-892
[14] Messer, W.B., U.T., Vitarana, K., Sivanathan, J., Elvitgala, L.D., Preethimala, R., Ramesh, N., Withana, D.J., Gubler, A.D, Silva. 2002. Epidemiology of dengue in Sri Lanka before and after the emergence of epidemic dengue hemorrhagic fever. American Journal of Tropical Medicine and Hygiene. 66(6):765–773
[15] Leitmeyer, K.C., D.W., Vaughn, D.M., Watts, R., Salas, I., Villalobos de Chacon, C., Ramos, R, Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. Journal of Virology. 73(6):4738-4747
[17] Srikiatkhachron, A. 2009. Plasma leakage in dengue haemorrhagic fever. Thromb. Haemost.. 102(6):1042-1049
[18] 2010. Letter to the Editor: sylvatic dengue viruses share the pathogenic potential of urban/endemic dengue viruses.Journal of Virology. 84(7):3726-3728
[19] Ooi, E., D.J., Gubler. 2008. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention.Cad. Saude Publica, Rio de Janeiro. 1:S115-S124
[20]Honorio, N.A., R.M.R., Nogueira, C.T., Codeco, M.S., Carvalho, O.G., Cruz, M., de Avelar Figueiredo Marfa Magalhaes, J.M., Galvo de Araujo, E.S., Machado de Araujo, M.Q., Gomes, L.S., Pinheiro, C.D.S., Pinel, R., Lourenco-de-Olivera. 2009. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLOS, Neglected Tropical Disease. 3(11):1-11
[21]New approaches to structure-based discovery of dengue protease inhibitors. 2010. “Simple representation of Neuraminidase N1 virtual screening using WISDOM grid production environment.” Available: http://www.dsimb.inserm.fr/~debrevern/IDDT-2009_in_silico_issue/iddt_2009_in_silico_issue.php [Date visited: 12/5/10]
[22] Acosta, E.G., L.B., Talarico, E.B., Damonte. 2008. DENV attachment: multiple cell receptors? Future Virology. 3(5):471-479
[23] Cologna, R., P.M., Armstrong, R., Rico-Hesse. 2004. Selection for virulent dengue viruses occurs in humans and mosquitoes Journal of Virology. 79(2):853-859
[24] Sanchez-Vargas, I., J.C., Scott, B.K., Poole-smith, A.W.E., Franz, V., Barbosa-Solomieu, J., Wilusz, K.E., Olson, C.D., Blair. 2009. Dengue viruse type 2 infections of Aedes aegypti are modulated by the mosquitoe’s RNA interference pathway. PLOS, Pathogens. 5(2):1-11
[25] Xi, Z., J.L., Ramirez, G., Dimpopoulos. 2008. The Aedes aegypti Toll pathway control dengue virus infection. PLOS, Pathogens. 4(7):1-11
Page authored for BIOL 375 Virology, September 2008