Fungal Endophytes: Drought Tolerance in Plants: Difference between revisions
SBarnes7151 (talk | contribs) No edit summary |
SBarnes7151 (talk | contribs) No edit summary |
||
| Line 20: | Line 20: | ||
==Reactions to Drought Stress== | ==Reactions to Drought Stress== | ||
===Osmotic Ajustment=== | ===Osmotic Ajustment=== | ||
<! | <!-- | ||
http://link.springer.com/chapter/10.1007%2F978-3-642-32653-0_8 | http://link.springer.com/chapter/10.1007%2F978-3-642-32653-0_8 | ||
Osmotic Adjustment Under Drought Conditions Gregor J. Sanders, Stefan K. Arndt | Osmotic Adjustment Under Drought Conditions Gregor J. Sanders, Stefan K. Arndt | ||
Revision as of 15:22, 14 April 2015
It has been estimated that over 80% of terrestrial plants form a symbiotic association with fungi.[1] Species of fungi that reside within living plant tissue without causing symptoms of disease in their host are known as fungal endophytes.[4] Fungal endophytes colonize a variety of both monocot and eudicot plants which suggests that this symbiosis predated the monocot-dicot split that occurred 140-150 million years ago.[2] It is also hypothesized that it was phototroph-fungi associations that enabled plants to first colonize land.[3] This mutualistic association could have helped plants acclimate to new environmental stresses such as desiccation, increased exposure to solar radiation, and more extreme temperatures differences.[3]
Fungal endophytes remain an important component of today’s terrestrial ecosystems, and many enable their hosts to thrive in harsh environments. Studies show that fungal endophytes can enhance the drought, salt, and soil temperature tolerance of their host plant in addition to increasing resistance to parasitic fungi and herbivores.[4] With growing concerns about climate change and its effects on agriculture, learning about fungal endophyte conferred drought tolerance has become increasingly important. By influencing plant morphology, development, and physiological and biochemical responses to stress, fungal endophytes can induce mechanisms of drought avoidance, drought tolerance, and drought recovery in their hosts.[8]
Classes of Fungal Endophytes
Clavicipitaceous Endophytes
Class 1
Clavicipitaceous endophytes are associated with grasses.[4] They typically are found within the plant shoots and form systemic intercellular infections.[4] While they are often passed down in the seed through vertical transmission, they may also undergo horizontal transmission.[4] Many class 1 endophytes produce alkaloids to protect their host plant from herbivory by insects and mammals, and studies have shown some class 1 endophytes to confer drought and metal tolerance.[5][8] Neotyphodium coenophialum , for example, stimulates plants to develop more extensive root systems and longer and thinner root hairs. [8]
Nonclavicipitaceous Endophytes
Nonclavicipitaceous endophytes are highly diverse and have been isolated from every major lineage of land plant, which includes nonvascular plants, ferns, conifers, and angiosperms.
Class 2
Class 2 endophytes are usually found in the roots, stem, or leaves of their hosts.[4] Like class 1 endophytes, they can also be transmitted either vertically through the seed coat or horizontally. [4] They are unique in that they can confer habitat-specific stress tolerance to their hosts.[4] They often increase root and/or shoot biomass in their host, and they infect a higher percentage of plants in high-stress environments.[4]
Figure 1. Four stages of root colonization by class 2 endophyte P. indica . 1. Extracellular colonization of the root surface 2. The hyphae penetrate the epidermal (RC) and cortical (C) cells. Cell organelles (e.g. nucleus; blue) remain intact and plasma membrane invaginate (dark gray lines inside cells). 3. The plasma membrane still surrounds intracellular hyphae while biotrophically colonized cells die (light gray filling of cells) and organelles are disrupted. 4. Fungal reproduction in RC and C cells. Endodermis cells (E) are not colonized. [12]
Class 3 and 4
Class 3 colonizes the shoot of plants while Class 4 colonizes plant roots.[4] Both are horizontally transmitted.[4] Class 3 endophytes are highly localized when they colonize a plant, and a diverse number of species can colonize an individual plant.[4] Few studies have been performed on Classes 3 and 4 endophytes, and little is known about their ecological role or their ability to confer tolerance.
Reactions to Drought Stress
Osmotic Ajustment
Redman et al (2015) examined the osmotic concentrations in non symbiotic and heat-stress tolerant symbiotic plants. The pattern was different between the two groups, leading them to conclude that symbiotic plants do not only rely on increasing their osmolyte concentrations.[6] Endophyte known to promote drought tolerance have high levels of loline alkaloids. [7] Future experiments could test if these are present in sufficient concentration to prevent the denaturation of macromolecules or reduce the number of reactive oxygen species. [7
Reactive Oxygen Species
Abiotic stresses such as drought result in the overproduction of reactive oxygen species (ROS) [10] These highly reactive metabolic products act as signaling molecules; however, when their levels are too high, they cause oxidative stress and damage proteins, lipids, and DNA. [10] [11] ROS control many plant processes such as growth, abiotic stress response, cell cycle, and programmed cell death because they influence the expression of genes. [10]
Figure 2. Signaling pathways of abiotic and biotic stress at the cellular level. Both types of stress factors affect the homeostasis of chemical signals such as Ca2+, ROS, and pH levels. Changes in these levels due to abiotic stress can affect the integrity of physical barriers against pathogens. Both abiotic and biotic stresses also share signaling nodes such as RBOHs (which produce ROS), MAPKs (signal cascades that regulate stress and hormonal responses), RLKs and other cell wall (CW) kinases which are used to regulate gene expression. The stress hormone Abscisic acid (ABA) signaling is central to abiotic stress response. It inhibits hormones responsible for growth and development such as auxin (AUX), cytokinin (CK) and gibberellin (GA) and triggers increased generation of ROS as well as plant defense signaling (JA). Redox state and metabolite concentrations of sugars and amino acids (AA) regulate post-translational modifications. Changes in chromatin and DNA methylation status alter the expression patterns of genes through transcription factor activation and its binding to stress responsive gene promoters. [13]
<!—
Symbiotically derived benefits
Arachevaleta Tall Fescue Endophyte 1989 Endopyte plants grew better after drought stress. May be affected by ccarbohydrate metabolism. Higher auxin production by endophyte (Porter et al 1985) could increase efficieny of mobilization and translocation of reserve cabohydrates which may be more important than actual levels. Cabohydrate percentages vary inversely with soil moisture levels because current leaf area is insufficient to produce energy needed for growth.
Latch et al (1985) 44% higher root biomass production for WT Infected ryegrass plants at low temp. Also increased root biomass in tall fescue (De Battista et al., 1990), and meadow fescue (Malinowski et al., 1997a, b). Malinowski et al. (1999b) showed that endophyte infection increased root hair length and decreased root diameter in tall fescue. These traits could increase root surface area for water and mineral acquisition.
More rapid stomatal closure in tall fescue and meadow fescue which reduces water loss through transpiration [8]. Biochemical signal from endopyte or changed hormonal status in plant predisposes leaves to respond quickly to water deficit Mutual defence responses involving secondary metabolites produced in higher quantities Endophyte may be an internal stress that sensitizes plant to other stresses.
Belesky et all (1989), Kelrick et al (1990), and De Battista et al (1990) all show similar in tall fescue Kelrick et al (1990) increased r-s ratio in I when water stressed and decreased r/s ratio in water stressed F Elmi et al (1990) higher osmotic adjustment in I than f tall fescue
Knox and Karnock 1992 Root and shoot growth of endophyte inflected and endophyte free tall fescue Kelrick et al (1993) Direcet interactions between infected and uninfected individuals of Festuca arundinacea: Differential allocation to shoot and root biomass
"some endophytes avoid stress through plant symbiosis. For example, Curvularia protuberata colonizes all nonembryonic tissues of the geothermal plant Dichanthelium lanuginosum (Redman et al., 2002; Márquez et al., 2007). When grown nonsymbiotically, neither the plant nor the fungus can tolerate temperatures above 40°C. However, the symbiosis allows both partners to tolerate temperatures up to 65°C. A similar scenario was observed with Fusarium culmorum which colonizes all nonembryonic tissues of coastal dunegrass ( Leymus mollis): when grown nonsymbiotically, the host plant does not survive and the endophyte’s growth is retarded when exposed to levels of salinity experienced in their native habitat (Rodriguez et al., 2008)." [4]
"most, if not all, of the Class 2 endophytes examined to date increase host shoot and/or root biomass, possibly as a result of the induction of plant hormones by the host or biosynthesis of plant hormones by the fungi (Tudzynski & Sharon, 2002). Many Class 2 endophytes protect hosts to some extent against fungal pathogens (Danielsen & Jensen, 1999; Narisawa et al., 2002; Campanile et al., 2007), reflecting the production of secondary metabolites (Schulz et al., 1999), fungal parasitism (Samuels et al., 2000), or induction of systemic resistance (Vu et al., 2006)."[4] -->
Testing Endophyte Conferred Tolerances
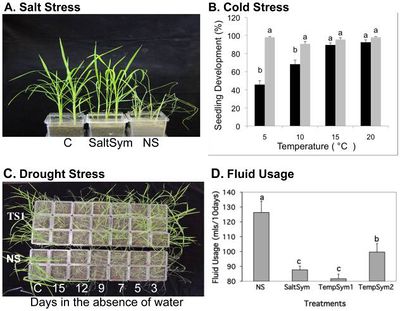
Redman et al (2015) tested how well the class 2 fungal endophytes Fusarium culmorum (SaltSym) and Curvularia protuberant (TempSym) would confer tolerance to salt, drought and cold to rice plants. SaltSym was isolated from the coastal plant Leymus mollis which is exposed to high salt stress, while TempSym 1 and 2 were isolated from Dichanthelium lanuginosum which grows in geothermal soils.
The study found that plants inoculated with the endophytes (S) showed no cost when grown in non-stressful conditions, but the number of colonized plants decreased from 100% to 65%.[6] When infected plants were grown in stressful environments, their water consumption decreased by 20–30% while their growth rate, reproductive yield, and biomass increased (Figure 1). [6] Non-infected plants (NS), on the other hand, lost shoot and root biomass when exposed to stress. All three endophytes treatments took 2-3 times longer to wilt than the non-infected; however, the mechanism of the conferred drought tolerance remains unknown. An interesting observation was that the endophytes changed the development of the plants to increase root biomass before shoot growth.
All plant species used in this experiment are members of the family Poacea but belong to different subfamilies. [6] The isolated fungal endophytes successfully conferred drought tolerance to the rice plants which supports the idea that the symbiotic communication needed to communicate between the fungi and the plant was conserved within the family. [6] While many fungal endophytes show habitat-adapted symbiosis, the fact that there is still lower biodiversity in high stress environments indicates that having the endophyte itself is not enough. [6]
Further Reading
References
|[1] [Smith, S., Read, D., 1997: Mycorrhizal symbiosis, 2nd edn., Academy Press, San Diego. ]
|[2] Shu-Miaw C., Chien-Chang C., Hsin-Liang C., Wen-Hsiung L."Dating the Monocot–Dicot Divergence and the Origin of Core Eudicots Using Whole Chloroplast Genomes". "Journal of Molecular Evolution". 2004. Volume 58, p. 424-441
|[3] Selosse, M-A, and F. Le Tacon. "The Land Flora: A Phototroph-Fungus Partnership?" Trends in Ecology & Evolution 13.1 (1998): 15-20. Print.
|[4] Rodriguez, R. J., et al. "Fungal Endophytes: Diversity and Functional Roles." New Phytologist 182.2 (2009): 314-30. Print.
|[5] Koulman, Albert, et al. "Peramine and Other Fungal Alkaloids are Exuded in the Guttation Fluid of Endophyte-Infected Grasses." Phytochemistry 68.3 (2007): 355-60. Print.
|[6] Redman, Regina S. et al. “Increased Fitness of Rice Plants to Abiotic Stress Via Habitat Adapted Symbiosis: A Strategy for Mitigating Impacts of Climate Change.” Ed. Hany A. El-Shemy. PLoS ONE 6.7 (2011): e14823. PMC. Web. 24 Mar. 2015.
|[7] Schardl, C. L., Leuchtmann, A. & Spiering, M. J. (2004) Annu. Rev. Plant Biol. 55, 315-340.
|[8] Malinowski DP, Belesky DP. 2000. Adaptations of endophtye-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science 40: 923–940.
|[9] Cheplick GP. 2006. Costs of fungal endophyte infection in Lolium perenne genotypes from eurasia and north africa under extreme resource limitation. Environmental and Experimental Botany 60: 202–210.
|[10] Gill, S, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909-930
|[11] Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I. and Laloi, C. (2006), Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays, 28: 1091–1101.
|[12] [1] Jacobs, S., Zechmann, B., Molitor, A., Trujillo, M., Petutschnig, E., Likpa, V., Kogel, K-H., Schaefer, P. (2011). Broad-spectrum suppression of innate immunity is required for colonization of arabidopsis roots by the fungus piriformospora indica. Plant Physiology, 156(2), 726-740. doi:10.1104/pp.111.176446
|[13] [2] Kissoudis, C., van de Wiel, C., Visser, R. G. F., & van der Linden, G. (2014). Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Frontiers in Plant Science, 5, 207. doi:10.3389/fpls.2014.00207
Edited by Sarah Barnes, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.
