Galdieria sulphuraria: Difference between revisions
No edit summary |
|||
| (46 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
[[Image:Gsulphcell.jpg|thumb|300px|right|Image of ''Galdiera sulphuraria'' courtesy of [http://www.nationalgeographic.com National Geographic.]]] | [[Image:Gsulphcell.jpg|thumb|300px|right|Image of ''Galdiera sulphuraria'' courtesy of [http://www.nationalgeographic.com National Geographic.]]] | ||
| Line 6: | Line 7: | ||
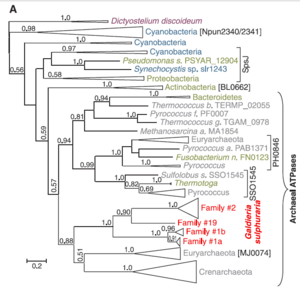
[[Image:Gsulphatpasecladogram.png|thumb|300px|left|Phylogeny of ATPases in ''G. sulphuria'' Gerald Schönknecht et al. [''Science'' 339, 1207 (2013).]]] | [[Image:Gsulphatpasecladogram.png|thumb|300px|left|Phylogeny of ATPases in ''G. sulphuria'' Gerald Schönknecht et al. [''Science'' 339, 1207 (2013).]]] | ||
===Species=== | ===Species=== | ||
Species: '' | Species: ''Galdieria sulphuraria'' | ||
==Description and Significance== | ==Description and Significance== | ||
Galdieria sulphuraria is | ''Galdieria sulphuraria'' is an extremophilic, spherical, spore-forming, eukaryotic red alga. ''G. sulphuraria'' appears yellow-green to dark blue-green grown heterotrophically in liquid culture, and often yellow or green in its natural environment[7]. It is an acidophile, as well a thermophile, and inhabits highly acidic springs at high temperatures[4]. | ||
''G. sulphuraria'' is a mixotrophic organism capable of both photosynthesis and the catabolism of a wide variety of metabolites[ | ''G. sulphuraria'' is a mixotrophic organism capable of both photosynthesis and the catabolism of a wide variety of metabolites[4]. Its ability to survive in extreme environment as well as its production of phycocyanin (PC) have made ''G. sulphuraria'' a candidate for biotechnology applications in both bioremediation and PC production [1,6]. | ||
==Genome Structure== | ==Genome Structure== | ||
''G. sulphuraria'' has | ''G. sulphuraria'' has 2 chromosomes composed of 9.8-13.7 Mbp, among the smallest of phototrophic eukaryotes [4,9] and a G+C concentration of 37% [5]. Phylogenetic and genomic analyses supplied by, Gerald Schönknecht et al. revealed 75 indications of horizontal gene transfer from archaea and bacteria along with highly condensed protein coding regions within the ''G. sulphuraria'' genome [4]. It is speculated that minimally 5% of the functional genes were acquired in this way [3]. | ||
Success of ''G. sulphuraria'' in a diverse array of extreme habitats appears to be facilitated by the acquisition and subsequent duplication of variety of genes not limited to heat tolerant archael ATPases, bacterial halophilic sodium-proton antiporters and thermoacidophilic arsenical membrane protein pumps along with the metal reducing mercuric reductase native to proteobacteria [ | Success of ''G. sulphuraria'' in a diverse array of extreme habitats appears to be facilitated by the acquisition and subsequent duplication of variety of genes not limited to heat tolerant archael ATPases, bacterial halophilic sodium-proton antiporters and thermoacidophilic arsenical membrane protein pumps along with the metal reducing mercuric reductase native to proteobacteria [4]. | ||
In addition to extremophilic adaptations, fungal metabolite transporters contribute to ''G. sulphuria's'' ability to utilize a medley of unusual carbon sources and distinguish it genetically even from "closely" related species [ | In addition to extremophilic adaptations, fungal metabolite transporters contribute to ''G. sulphuria's'' ability to utilize a medley of unusual carbon sources and distinguish it genetically even from "closely" related species [4]. | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
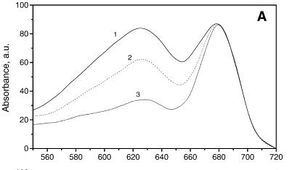
[[Image:Absorbtion_Spectrum.jpg|thumb|300px|left| | [[Image:Absorbtion_Spectrum.jpg|thumb|300px|left|Absorption spectrum of G. sulphuraria, Igor N. Stadnichuk et al. Biochimica et Biophysica Acta(BBA) - Bioenergetics 1807 (2011): 227–235]] | ||
''G. sulphuraria'' is a mixotrophic eukaryote presenting a multilobed chloroplast and net-like mitochondrion, is roughly round in shape, and reproduces via the formation of 4-32 spores[2]. | ''G. sulphuraria'' is a mixotrophic eukaryote presenting a multilobed chloroplast and net-like mitochondrion, is roughly round in shape, and reproduces via the formation of 4-32 spores[2]. | ||
''G. sulphuraria'' is capable of metabolic flexability, with the ability to grow photoautotrophically, photoheterotrophically, or chemoheterotrophically on greater than 50 carbon sources [ | ''G. sulphuraria'' is capable of metabolic flexability, with the ability to grow photoautotrophically, photoheterotrophically, or chemoheterotrophically on greater than 50 carbon sources [4]. Experiments in growing ''G. sulphuraria'' in laboratory conditions conditions determined that ''G. sulphuraria'' growth is optimal at about 150 g/liter of glucose or fructose, growth is inhibited above 200 g/liter, and does not occur above 400 g/liter [3]. Grown autotrophically, the photosystems of ''G. sulphuraria'' have the absorption spectra on the left, with peaks at the ~620 and ~680nm wavelengths[6]. | ||
Adaptations to an acidic, metal rich environment are essential to the survival of ''G. sulphuraria''. In order to deal with high heavy metal concentrations in its environment, ''G. sulphuraria'' has evolved a number of metal transporters in order to keep intracellular concentrations low, while allowing the selective uptake of metals needed for growth [4]. It is also capable of neutralizing biohazardous metals in some instances [4]. To maintain internal pH, ''G. sulphuraria'' has a lower H+ permeability than most eukaryotes, and only one voltage-gated ion channel gene. To maintain membrane voltage, ''G. sulphuraria'' contains more proteins in the intracellular chloride channel family and chloride carrier/channel family, as well as several Na+/H+ antiporters [4]. | |||
of | |||
==Ecology== | ==Ecology== | ||
[[Image:Gsulphhabitat.jpg|thumb|300px|right|Image of ''Galdieria sulphuraria'' in Reykjavik http://www.nationalgeographic.com National Geographic.]] | [[Image:Gsulphhabitat.jpg|thumb|300px|right|Image of ''Galdieria sulphuraria'' in Reykjavik http://www.nationalgeographic.com National Geographic.]] | ||
''G. sulphuraria'' is found in extreme habitats such as the hot sulfur springs of Italy, Russia, Yellowstone park, and Iceland in pH values between 0 and 4 and in temperatures up to 56 degrees Celsius [ | ''G. sulphuraria'' is found in extreme habitats such as the hot sulfur springs of Italy, Russia, Yellowstone park, and Iceland in pH values between 0 and 4 and in temperatures up to 56 degrees Celsius [7]. The high heavy metal concentrations found in many of these environments have also necessitated the evolution of adaptations to tolerate these conditions[4]. | ||
''G. sulphuraria'' can make up up to 90% of the total microbial mass in some environments [ | ''G. sulphuraria'' can make up up to 90% of the total microbial mass in some environments [3], however it often exists alongside ''Cyanidioschyzon merolae'' and ''Cyanidium caldarium'' [5]. | ||
==Applications to Biotechnology== | ==Applications to Biotechnology== | ||
As mentioned in the Description and Significance section, ''G. sulphuraria'' has possible uses in both bioremediation and production of phycocyanin (PC) [ | As mentioned in the Description and Significance section, ''G. sulphuraria'' has possible uses in both bioremediation and production of phycocyanin (PC) [1,8]. In the case of PC, ''G. sulphuraria'' strain 074G grown on glucose produces 20-287 times the amount of PC produced by ''Spirulina platensis'', currently used in commercial PC production. Another advantage of ''G. sulphuraria'' is that it produces PC aphotically whereas ''S. platensis'' requires light for PC production, making potential commercial production easier [1]. ''G. sulphuraria'' is also a potential candidate for future applications in bioremediation due to its ability ability to neutralize some biohazardous metals [4]. | ||
==References== | ==References== | ||
[1] http:// | [1] [http://onlinelibrary.wiley.com/doi/10.1002/bit.20417/pdf Ankerstjerne Schmidt, Rikke et al. Heterotrophic High Cell-Density Fed-Batch Cultures of the Phycocyanin-Producing Red Alga ''Galdieria sulphuraria''. Biotechnology and Bioengineering 90 (2005): 77-84.] | ||
[ | [2] [http://link.springer.com/content/pdf/10.1023%2FA%3A1004035224715.pdf Cozzolino, Salvatore et al. Molecular variation in Galdieria sulphuraria (Galdieri) Merola and its bearing on taxonomy. Hydrobiologia 433 (2000):145–151.] | ||
[ | [3] [http://link.springer.com/content/pdf/10.1007%2Fs00253-007-1150-2.pdf Graverholt, Olav Sune and Niels Thomas Eriksen. Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Applied Microbiology and Biotechnology 77 (2007):69-75.] | ||
[ | [4] [http://www.sciencemag.org/content/339/6124/1207.full.html Schönknecht, Gerald et al. Gene Transfer from Bacteria and Archaea Facilitated Evolution of an Extremophilic Eukaryote. Science 339 (2013): 1207-1209.] | ||
[ | [5] [http://books.google.com/books?id=FIpKlCoSAYUC&pg=PA497&lpg=PA497&dq=G.+Sulphuraria+first+described&source=bl&ots=xwAfsGLnZc&sig=8KRUvBsjsZY1xtIImTN-vkALcIQ&hl=en&sa=X&ei=4HJrUe3JLKmY2wWRn4HQCw&ved=0CGsQ6AEwCDgK#v=onepage&q=G.%20Sulphuraria%20first%20described&f=false Seckbach, Joseph. Algae and Cyanobacteria in Extreme Environments. 2007.] | ||
[ | [6] [http://www.sciencedirect.com/science/article/pii/S0005272810007267#gr1 Stadnichuk, Igor et al. Far-red light-regulated efficient energy transfer from phycobilisomes to photosystem I in the red microalga ''Galdieria sulphuraria'' and photosystems-related heterogeneity of phycobilisome population. Biochimica et Biophysica Acta(BBA) - Bioenergetics 1807 (2011): 227–235] | ||
[ | [7] [http://genomics.msu.edu/galdieria/about.html The ''Galdieria sulphuraria'' Genome Project] | ||
[ | [8] [http://www.ncbi.nlm.nih.gov/pubmed/15604662 Weber, A. et al. EST-analysis of the thermo-acidophilic red microalga Galdieria sulphuraria reveals potential for lipid A biosynthesis and unveils the pathway of carbon export from rhodoplasts. Plant Molecular Biology 55 (2004): 17-32.] | ||
[ | [9] [http://journals.cambridge.org/abstract_S0967026201003225 Muravenko, O. et al. Chromosome numbers and nuclear DNA contents in the red microalgae ''Cyanidium caldarium'' and three Galdieria species. European Journal of Phycology 36 (2001):27-232] | ||
==Author== | ==Author== | ||
By Mark Johnson and Brandon Cohn. | |||
Latest revision as of 13:29, 1 October 2015

Classification
Domain: Eukaryota; Class: Rhodophyta; Family: Cyanidiaceae; Genus: Galdieria
Species
Species: Galdieria sulphuraria
Description and Significance
Galdieria sulphuraria is an extremophilic, spherical, spore-forming, eukaryotic red alga. G. sulphuraria appears yellow-green to dark blue-green grown heterotrophically in liquid culture, and often yellow or green in its natural environment[7]. It is an acidophile, as well a thermophile, and inhabits highly acidic springs at high temperatures[4].
G. sulphuraria is a mixotrophic organism capable of both photosynthesis and the catabolism of a wide variety of metabolites[4]. Its ability to survive in extreme environment as well as its production of phycocyanin (PC) have made G. sulphuraria a candidate for biotechnology applications in both bioremediation and PC production [1,6].
Genome Structure
G. sulphuraria has 2 chromosomes composed of 9.8-13.7 Mbp, among the smallest of phototrophic eukaryotes [4,9] and a G+C concentration of 37% [5]. Phylogenetic and genomic analyses supplied by, Gerald Schönknecht et al. revealed 75 indications of horizontal gene transfer from archaea and bacteria along with highly condensed protein coding regions within the G. sulphuraria genome [4]. It is speculated that minimally 5% of the functional genes were acquired in this way [3].
Success of G. sulphuraria in a diverse array of extreme habitats appears to be facilitated by the acquisition and subsequent duplication of variety of genes not limited to heat tolerant archael ATPases, bacterial halophilic sodium-proton antiporters and thermoacidophilic arsenical membrane protein pumps along with the metal reducing mercuric reductase native to proteobacteria [4].
In addition to extremophilic adaptations, fungal metabolite transporters contribute to G. sulphuria's ability to utilize a medley of unusual carbon sources and distinguish it genetically even from "closely" related species [4].
Cell Structure, Metabolism and Life Cycle
G. sulphuraria is a mixotrophic eukaryote presenting a multilobed chloroplast and net-like mitochondrion, is roughly round in shape, and reproduces via the formation of 4-32 spores[2].
G. sulphuraria is capable of metabolic flexability, with the ability to grow photoautotrophically, photoheterotrophically, or chemoheterotrophically on greater than 50 carbon sources [4]. Experiments in growing G. sulphuraria in laboratory conditions conditions determined that G. sulphuraria growth is optimal at about 150 g/liter of glucose or fructose, growth is inhibited above 200 g/liter, and does not occur above 400 g/liter [3]. Grown autotrophically, the photosystems of G. sulphuraria have the absorption spectra on the left, with peaks at the ~620 and ~680nm wavelengths[6].
Adaptations to an acidic, metal rich environment are essential to the survival of G. sulphuraria. In order to deal with high heavy metal concentrations in its environment, G. sulphuraria has evolved a number of metal transporters in order to keep intracellular concentrations low, while allowing the selective uptake of metals needed for growth [4]. It is also capable of neutralizing biohazardous metals in some instances [4]. To maintain internal pH, G. sulphuraria has a lower H+ permeability than most eukaryotes, and only one voltage-gated ion channel gene. To maintain membrane voltage, G. sulphuraria contains more proteins in the intracellular chloride channel family and chloride carrier/channel family, as well as several Na+/H+ antiporters [4].
Ecology

G. sulphuraria is found in extreme habitats such as the hot sulfur springs of Italy, Russia, Yellowstone park, and Iceland in pH values between 0 and 4 and in temperatures up to 56 degrees Celsius [7]. The high heavy metal concentrations found in many of these environments have also necessitated the evolution of adaptations to tolerate these conditions[4].
G. sulphuraria can make up up to 90% of the total microbial mass in some environments [3], however it often exists alongside Cyanidioschyzon merolae and Cyanidium caldarium [5].
Applications to Biotechnology
As mentioned in the Description and Significance section, G. sulphuraria has possible uses in both bioremediation and production of phycocyanin (PC) [1,8]. In the case of PC, G. sulphuraria strain 074G grown on glucose produces 20-287 times the amount of PC produced by Spirulina platensis, currently used in commercial PC production. Another advantage of G. sulphuraria is that it produces PC aphotically whereas S. platensis requires light for PC production, making potential commercial production easier [1]. G. sulphuraria is also a potential candidate for future applications in bioremediation due to its ability ability to neutralize some biohazardous metals [4].
References
[5] Seckbach, Joseph. Algae and Cyanobacteria in Extreme Environments. 2007.
[7] The Galdieria sulphuraria Genome Project
Author
By Mark Johnson and Brandon Cohn.