Gordonia
Classification
Higher order taxa
Kingdom; Phylum; Class; Order; Family; Genus [Organism&cmd=DetailsSearch]
Description and significance
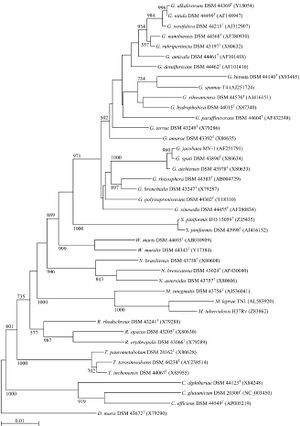
The genus of Gordonia was originally proposed as Gordona 1971 by Tsukamora M for a bacterium isolated from the spit of patients with pulmonary disease and soil. It was named Gordona to pay tribute to pay tribute to an American Bacteriologist Ruth E. Gordon. This genus was eventually discarded then reinstated later and in 1997 it was renamed Gordonia.
One of the most significant things about these bacteria is that they has the ability to degrade some xenobiotics, pollutants, and some other slow to biodegrade products. They also have applications in biotechnology with their ability to synthesize potentially useful compounds. The fact that many species of Gordonia are opportunistic pathogens, limits this application though.
Genome structure
The median genome length of Gordoniae is 5.27 mB.
The median GC content is 67.4%.
Cell structure and metabolism
Gordonia members are characterized as aerobic, nonmotile bacteria that can be gram-positive to gram-variable actinomycetes. They are also catalase-positive, slightly acid-fast, with some apparent susceptibility to lysozyme. Because the Gordoniae resemble the Nocardiae, the Gordoniae are referred to as nocardioforms. This means that their mycelial growth fragments into bacillar to coccoid filaments. No spores are produced. (2)
Metabolically, Gordoniae are oxidative with a preference for breaking down carbohydrates for energy. Although a wide variety of other substances can be broken down as well, Gordoniae are arylsulfatase negative. (2)
Colony morphology has great variety even within the same species (such as Gordonia alkanivorans DSM 44369 and Gordonia westfalica DSM 44215) or the medium used. Colonies range from slimy, smooth, glossy, irregular, and rough with colors spanning white, tannish, yellow, orange, red, and pink. The morphology of colonies appears able to be manipulated. In particular, smooth colonies can be irreversibly converted into rough ones by causing mutations in the genes encoding methyltransferases involved in glycopeptidolipid synthesis. (2)
The cell wall of Gordoniae use arabinose, galactose, and glucose. The sugars of the peptidoglycan are glycolated. The only diamino acid of the peptidoglycan is meso-diaminopimelic acid. The peptidoglycan also includes muramic acid with N-glycolyl residues, meaning that the peptidoglycan is of A1 γ type. The cell wall is categorized as chemotype IV sensu Lechevalier and Lechevalier. The polar lipids are typically composed of diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylinositol, and phosphatidylinositol mannosides. MK-9(H2) is the dominant menaquinone/isoprenologue. MK-8(H2) is only found in traces of some species. These cell wall and cellular chemotaxonomic properties separate Gordonia from related genera such as Mycrobacterium, Rhodococcus, and Skermania. (2) (9)
Ecology
The Gordonia genus includes a variety of versatile species that have been isolated from multiple types of environments. (9) Gordonia have been found terrestrially in soil and mangrove rhizospheres. (2) They have also been found in aquatic environments including both marine and freshwater ecosystems. (9) Gordonia have been specifically isolated from estuary sands as well. (5) Because Gordonia are highly capable of breaking down waste products, they have been largely grown in oil-producing wells, hydrocarbon-contaminated soil, wastewater treatment plants, bioreactors and biofilters, and activated sludge. (2) (5) Gordonia are also found to have symbiotic relations with multiple hosts in marine and freshwater environments as well as terrestrial invertebrates. (9) A few species such as G. aichiensis, G. araii, G. bronchialis, G. effusa, and G. sputi were actually originally isolated from clinical specimens, so it is not uncommon for Gordonia species to be found inhabiting humans, too. (5)
Applications to Biotechnology
The members of the genus Gordonia are very diverse in their abilities to degrade various hydrocarbons, pollutants, xenobiotics, and natural compounds that are not readily biodegradable. This makes the Gordoniae very good candidates for bioremediation, however past infections in high-risk individuals may limit their use. Some molecules and conpunds that can be broken down are phthalic acid esters, s-triazine and alkylpyridines, DBT and biodesulfurization, other xenobiotics, and natural and synthetic rubbers. (2)
The Gordoniae can also produce different compounds either exclusively or as an alternative such as: biosurfactants, carotenoids, imidazol-2-yl amino acids, gordonan, gordonin. (2)
Current Research
Many newly isolated and characterized Gordonia strains have attracted researchers in recent years due to their abilities to degrade environmental pollutants such as polycyclic aromatic hydrocarbons (PAHs), alkylpyridines, phthalates, xenobiotic compounds (e.g. 1,3,5-triazines such as RDX) or slowly biodegradable natural polymers (e.g. rubber). (4) To list some examples of the unusual and diverse metabolic capabilities of Gordoniae, three strains of G. polyisoprenivorans and the novel species G. westfalica Kb1 were able to degrade natural rubber and synthetic cis-1,4-polyisoprene, which allows species of this genus to serve as model organisms for the investigation of the unknown biochemical and molecular mechanisms of rubber biodegradation. (3) While the Gordoniae have been proven useful in degrading and synthesizing many compounds, the problem is that some Gordoniae are opportunistic pathogens. In order to use the genes of the Gordoniae for environmental and industrial biotechnology, there has been research done to genetically transfer the phenotypic features of Gordoniae to other microorganisms. Recent studies have revealed improved cloning vectors allowing the transfer of Gordonia genes to E. coli. (2) The G. nitida dszABC genes for desulfurization were expressed in several different E. coli strains under an inducible trc promoter. Cultivation of these metabolically engineered E. coli strains in the presence of 0.2 mM dibenzothiophene (DBT) allowed the conversion of DBT to 2-hydroxybiphenyl (2-HBP), which is the final metabolite of the sulfur-specific desulfurization pathway. The maximum conversion of DBT to 2-HBP was 16% in 60 hours. Recombinant E. coli was applied for the deep desulfurization of diesel oil supplemented into the medium at 5% (v/v). Sulfur content in diesel oil was decreased from 250 mg sulfur/1 to 212.5 mg sulfur/1, resulting in the removal of 15% of sulfur in diesel oil in 60 hours. (7)
Additionally, Gordoniae have shown the ability to transform and synthesize organic compounds such as canthaxanthin that can be useful for medical applications. Canthaxanthin is used to reduce sensitivity to sunlight experienced by people with the rare disease erythropoietic protoporphyria (EPP). People with this condition experience skin reactions such as rash, itch, and eczema when exposed to sunlight. (10) If Gordoniae are able to industrially produce canthaxanthin, this could be a revolutionary treatment for people with EPP. In the food industry, canthaxanthin is used as food coloring and is added to animal feed to improve the color of chicken skins, egg yolks, salmon, and trout. Canthaxanthin is also used in some cosmetics. (10) Gordoniae producing canthaxanthin could become a very cost-effective way to manufacture many products in the medical, cosmetic, and food industries.
Interesting Features
Some Gordonia species isolated from clinical specimens are known to be opportunistic human pathogens causing secondary infections in immunocompromised and immunosuppressive individuals. (9) Altogether, approximately 20 case reports of Gordonial infections can be found in the literature; most of these infections were caused by G. bronchialis and were associated with sternal wounds resulting from surgery. A few infections also occurred after coronary artery bypass surgery and were associated with heart-lung machines or applications of central venous or Hickman catheters. (2) Infections caused by other Rhodococcus spp. and related genera, such as Gordonia and Tsukamurella, have generally been associated with medical procedures or devices. Gordonia spp., previously classified as Rhodococcus spp., have caused bacteremia, endocarditis, and CNS infections in both immunocompromised and immunocompetent adults and children. Gordonia terrae has caused central venous catheter-associated bacteremia and endocarditis in children and infections of medical devices. Gordonia bronchialis (formerly Rhodococcus bronchialis) has been reported to cause a cluster of sternal wound infections after coronary artery bypass surgery, bacteremia, osteomyelitis, pleural infection, and recurrent breast abscess. Pulmonary infection resembling tuberculosis has been reported to be caused by Gordonia rubripertinctaus (formerly Rhodococcus rubropertinctus) in a patient who was not immunosuppressed. (8)
References
To be added