H1N1: Difference between revisions
No edit summary |
No edit summary |
||
| (22 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
=Introduction= | =Introduction= | ||
[[Image:Swine flu blob.jpg|thumb|300px|right|Swine Flu Virus]] | |||
Starting in April, 2009, a wide-spread epidemic infected the world’s population and perhaps even more so, infected the media. According to a study by Ding et al. (2009), H1N1 Influenza A virus, or better known as swine flu, made nearly 22,000 people ill and killed 125 of them by August, 2009. In all, by that time, the virus had spread through 69 countries and/or regions of the world (starting in Mexico). Ding et al. also explains that this specific strain of influenza is particularly concerning due to its uncommon ability to be passed from human to human (unlike most swine and avian A influenzas). While examining the mechanisms and characterization of the virus is important, incorporating these findings with the evolutionary capabilities and mutability of H1N1 novel influenza A represent an optimal way of examining the virus and potentially using this information to develop a cure for infected hosts. | |||
=Characterization of Influenza Viruses= | =Characterization of Influenza Viruses= | ||
| Line 8: | Line 24: | ||
[[Image:Swine_flu_virus_cross_section_general.jpg|thumb|300px|right|Figure 1. Computer generated cut picture of single H1N1 virus particle with labeled proteins and receptors. Pb1 pb2 pa= polymerase genes, Ha= hemagglutinin, NP=nucleoprotein genes, Na=neuramindase. (http://www.supplementinfo.org/index.php?src=blog&srctype=detail&refno=186&category=Consumer)]] | [[Image:Swine_flu_virus_cross_section_general.jpg|thumb|300px|right|Figure 1. Computer generated cut picture of single H1N1 virus particle with labeled proteins and receptors. Pb1 pb2 pa= polymerase genes, Ha= hemagglutinin, NP=nucleoprotein genes, Na=neuramindase. (http://www.supplementinfo.org/index.php?src=blog&srctype=detail&refno=186&category=Consumer)]] | ||
Influenza A viruses, the type that are capable of human infection, are characterized by two proteins. More specifically, the glycoproteins hemagglutinin and neuraminidase are numbered based on their identified character and are the two methods of categorization. Hemagglutinin is labeled “H” and numbered 1-16 (H16 was discovered very recently) and can be differentiated by amino acid composition. Its viral function is to aid in host cell binding. Specifically, it links to sialic acid located on the surface of the targeted host cell. This greatly contributes to infection capability. The other element used for subtype categorization for influenza A is the neuraminidase glycoprotein. Its role in infection is similar to that of hemagglutinin, but neuraminidase cleaves the poly-sialic surface acids in order to allow for the exiting of viral “offspring.” Neuraminidase has nine different amino acid sequences and is thus labeled N1-N9. While there clearly are many combinations of the two glycoproteins that cooperate to infect a host cell, only three influenza A subtypes have been commonly found to successfully infect and transmit from human cell to human cell. This narrowing of possibilities has been crucial in fighting the biological war to control, vaccinate, and potentially someday cure the debillitating, and even sometimes fatal, virus in humans. | Influenza A viruses, the type that are capable of human infection, are characterized by two proteins. More specifically, the glycoproteins hemagglutinin and neuraminidase are numbered based on their identified character and are the two methods of categorization. Hemagglutinin is labeled “H” and numbered 1-16 (H16 was discovered very recently) and can be differentiated by amino acid composition (Article: "Hemagglutnin"). Its viral function is to aid in host cell binding. Specifically, it links to sialic acid located on the surface of the targeted host cell. This greatly contributes to infection capability. The other element used for subtype categorization for influenza A is the neuraminidase glycoprotein. Its role in infection is similar to that of hemagglutinin, but neuraminidase cleaves the poly-sialic surface acids in order to allow for the exiting of viral “offspring.” Neuraminidase has nine different amino acid sequences and is thus labeled N1-N9 (Al Farress et al. 2005). Figure 1 should be referenced to to observe the positioning and layout of the hemagglutinin and neuraminidase proteins on the virus. While there clearly are many combinations of the two glycoproteins that cooperate to infect a host cell, only three influenza A subtypes have been commonly found to successfully infect and transmit from human cell to human cell. This narrowing of possibilities has been crucial in fighting the biological war to control, vaccinate, and potentially someday cure the debillitating, and even sometimes fatal, virus in humans. | ||
==Hemagglutinin and Neuraminidase== | ==Hemagglutinin and Neuraminidase== | ||
| Line 16: | Line 32: | ||
A study by Al Farress et al. (2005) takes a look at the evolutionary aspect of these two glycoproteins. The study predicted that over time, based on the phylogeny of the hemagglutinin glycoprotein’s genetic origins that characterizes the type of influenza, there would be the possibility of so great a divergence between the antigenic characteristics of H1N1 and H1N2 influenza A subtypes that they could require significant changes to the vaccinations and treatments of the two strands of influenza. The implications of this conclusion and prediction by Al Faress et al.(2005) may already be felt in the form of novel H1N1 influenza A’s pandemic infection. This immediately brings to the forefront the necessity of the comprehension of the capabilities of such virus evolution and the possibilities of vaccination adaptation and thus resistance that they carry in order to prevent similar cases of influenza epidemic. | A study by Al Farress et al. (2005) takes a look at the evolutionary aspect of these two glycoproteins. The study predicted that over time, based on the phylogeny of the hemagglutinin glycoprotein’s genetic origins that characterizes the type of influenza, there would be the possibility of so great a divergence between the antigenic characteristics of H1N1 and H1N2 influenza A subtypes that they could require significant changes to the vaccinations and treatments of the two strands of influenza. The implications of this conclusion and prediction by Al Faress et al.(2005) may already be felt in the form of novel H1N1 influenza A’s pandemic infection. This immediately brings to the forefront the necessity of the comprehension of the capabilities of such virus evolution and the possibilities of vaccination adaptation and thus resistance that they carry in order to prevent similar cases of influenza epidemic. | ||
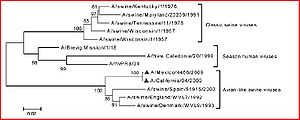
Samples of genetic regions that code for the hemagglutinin (HA 1 sequences-obtained from AH-1 strains) from three different time periods were isolated from humans experiencing influenza-like symptoms in the winters of 2001-2002, 2002-2003, and 2003-2004 in southern France. The genetic sequences were amplified by PCR. A bootstrap analysis was replicated 1000 times to perform a phylogenetic analysis. Likelihood of genetic distances was then calculated to verify the results. | Samples of genetic regions that code for the hemagglutinin (HA 1 sequences-obtained from AH-1 strains) from three different time periods were isolated from humans experiencing influenza-like symptoms in the winters of 2001-2002, 2002-2003, and 2003-2004 in southern France. The genetic sequences were amplified by PCR. A bootstrap analysis was replicated 1000 times to perform a phylogenetic analysis. Likelihood of genetic distances was then calculated to verify the results (Figure 2). | ||
=Evolution of Swine Flu= | =Evolution of Swine Flu= | ||
==H1N1 Is Product of Former Flu Viruses== | ==H1N1 Is Product of Former Flu Viruses: A Study by Ding et al.== | ||
[[Image:Swine_flu_virus_cross_section.gif |thumb|300px|right|Figure 3. Computer generated cross sectional picture showing the evolutionary roots of the different elements of an H1N1 virion. (http://www.flightsone.com/blog/)]] | [[Image:Swine_flu_virus_cross_section.gif |thumb|300px|right|Figure 3. Computer generated cross sectional picture showing the evolutionary roots of the different elements of an H1N1 virion. (http://www.flightsone.com/blog/)]] | ||
Phylogenetically, Ding et al. (2009) established that the novel swine flu has been found to originate in older viruses that were the product of reassortment of formerly triple-reassortant swine flu viruses. These older viruses were from a North American and Eurasian swine lineage that led to a lineage of avian and human roots. It has been further determined that six gene segments found in the novel swine flu virus that are close homologs to the aforementioned parent strands (polymerase PB2 (PB2), PB1, polymerase PA (PA), hemagglutinin (HA), nucleoprotein (NP), and nonstructural protein (NS)). The term swine flu is derived from the fact that it has long been proven that pigs are well-known vehicles for the mixing and production of new, hybrid versions of influenza viruses. It was thought that because of this, the triple reassortment that occurs in swine is derived from avian, swine, and human and passed on to human, causing a pandemic. By the time this study was published, it was found that there were no pigs infected with the novel influenza virus and that the name and theory was unfounded. The next hypothesis, is that there has been a cross-species contamination controlled by positive selection of novel H1N1 that enables the virus to use as a human as a host and transmit from human to human. The following study by Ding et al. (2009) performs adaptive and phylogenetic analyses to test this assertion. | Phylogenetically, Ding et al. (2009) established that the novel swine flu has been found to originate in older viruses that were the product of reassortment of formerly triple-reassortant swine flu viruses. These older viruses were from a North American and Eurasian swine lineage that led to a lineage of avian and human roots. It has been further determined that six gene segments found in the novel swine flu virus that are close homologs to the aforementioned parent strands (polymerase PB2 (PB2), PB1, polymerase PA (PA), hemagglutinin (HA), nucleoprotein (NP), and nonstructural protein (NS)). For the evolutionary origins of these individual proteins, see figure 3. The term swine flu is derived from the fact that it has long been proven that pigs are well-known vehicles for the mixing and production of new, hybrid versions of influenza viruses. It was thought that because of this, the triple reassortment that occurs in swine is derived from avian, swine, and human and passed on to human, causing a pandemic. By the time this study was published, it was found that there were no pigs infected with the novel influenza virus and that the name and theory was unfounded. The next hypothesis, is that there has been a cross-species contamination controlled by positive selection of novel H1N1 that enables the virus to use as a human as a host and transmit from human to human. The following study by Ding et al. (2009) performs adaptive and phylogenetic analyses to test this assertion. | ||
A few methods went came into play for testing this hypothesis. Sequences of novel A influenza derived from cases in 5 continents (excluding South American and Antarctica) and from the three different known possessing organisms: human, avian, and swine (served as a carrier, rather than an infected host). Because the HA gene in novel swine-originating flu were potentially closer homologies to A/H1N2 viruses than parent A/H1N1 viruses, A/H1N2 sequences were also obtained. For further analyses and to establish an outgroup, sequences of H5N1 and H3N2 were also retrieved. To actually perform the phylogenetic analysis, the experimenters used a program we are familiar with, CLUSTAL W (using the neighbor-joining method and 1000 replications to ensure reliability). In order to determine whether or not the novel H1N1 virus is driven by positive selection (to promote cross-species passing and human infection), the McDonald-Kreitman Test (MKT) was applied to test for natural selection of the virus. This test examines the ratio of nonsynonymous to synonymous polymorphisms in a species to detect diversity. It is compared to the neutrality index (NI), which is the ratio of nonsynonymous to synonymous fixed substitutions. It should be noted that nonsynonymous substitutions are base pair mutations where the genetic code is altered, and in synonymous mutations the code is preserved. Both novel H1N1 and background swine influenza A viruses were applied this test. CODEML was employed to confirm the results of MKT. This program detects selective pressure in the sequences aligned by CLUSTAL W. From these tests and methods, comparisons were made by bootstrap method. | A few methods went came into play for testing this hypothesis. Sequences of novel A influenza derived from cases in 5 continents (excluding South American and Antarctica) and from the three different known possessing organisms: human, avian, and swine (served as a carrier, rather than an infected host). Because the HA gene in novel swine-originating flu were potentially closer homologies to A/H1N2 viruses than parent A/H1N1 viruses, A/H1N2 sequences were also obtained. For further analyses and to establish an outgroup, sequences of H5N1 and H3N2 were also retrieved. To actually perform the phylogenetic analysis, the experimenters used a program we are familiar with, CLUSTAL W (using the neighbor-joining method and 1000 replications to ensure reliability). In order to determine whether or not the novel H1N1 virus is driven by positive selection (to promote cross-species passing and human infection), the McDonald-Kreitman Test (MKT) was applied to test for natural selection of the virus. This test examines the ratio of nonsynonymous to synonymous polymorphisms in a species to detect diversity. It is compared to the neutrality index (NI), which is the ratio of nonsynonymous to synonymous fixed substitutions. It should be noted that nonsynonymous substitutions are base pair mutations where the genetic code is altered and thus the amino acid residue is changed from that of the parental sequence, and in synonymous mutations the code is preserved. Both novel H1N1 and background swine influenza A viruses were applied this test. CODEML was employed to confirm the results of MKT. This program detects selective pressure in the sequences aligned by CLUSTAL W. From these tests and methods, comparisons were made by bootstrap method. | ||
All examined viruses were divided into two clades on eight phylogenetic trees (assumes great genetic divergence in older phases of H1N1). The HA, PB2, NP, and NS gene segments were found to be a trait common with avian and swine lineages, and also three human-based lineages that were able to spread amongst humans( seasonal human A/H1N1, 1918 Spanish A/H1N1, and the novel H1N1). Thus, researchers determined that the virus is capable of evolving the ability to pass from human to human, animal to animal, and across species. Furthermore, the aforementioned human strands of the virus have differing positions of certain gene segments, presenting the feasibility of independent evolution and reassortment (which yields the potential for the human-to-human transmission capabilities of the virus) of the segments. The phylogenetic trees of the eight tested genetic segments of the three mentioned human-based traits yield some interesting data. The Na and M segments are homologous to the segments of Eurasian swine influenza lineages, while the HA segment is most associated with the North American version. These results correlate with the hypothesis of cross-species passing that resulted in elements from triple-reassortant, Eurasia, and North American swine influenza strands. Other species of swine-based influenza have shown the ability to transfer cross-species and thus it is found that the virus has developed the significant ability to reassort. Also, secondary reassortments allow for the virus to adapt and infect human hosts. This raises significant warning signs because the potential for the virus to further reassort and cause future and more lethal infection in humans exists. | All examined viruses were divided into two clades on eight phylogenetic trees (assumes great genetic divergence in older phases of H1N1). The HA, PB2, NP, and NS gene segments were found to be a trait common with avian and swine lineages, and also three human-based lineages that were able to spread amongst humans( seasonal human A/H1N1, 1918 Spanish A/H1N1, and the novel H1N1). Thus, researchers determined that the virus is capable of evolving the ability to pass from human to human, animal to animal, and across species. Furthermore, the aforementioned human strands of the virus have differing positions of certain gene segments, presenting the feasibility of independent evolution and reassortment (which yields the potential for the human-to-human transmission capabilities of the virus) of the segments. The phylogenetic trees of the eight tested genetic segments of the three mentioned human-based traits yield some interesting data. The Na and M segments are homologous to the segments of Eurasian swine influenza lineages, while the HA segment is most associated with the North American version. These results correlate with the hypothesis of cross-species passing that resulted in elements from triple-reassortant, Eurasia, and North American swine influenza strands. Other species of swine-based influenza have shown the ability to transfer cross-species and thus it is found that the virus has developed the significant ability to reassort. Also, secondary reassortments allow for the virus to adapt and infect human hosts. This raises significant warning signs because the potential for the virus to further reassort and cause future and more lethal infection in humans exists. | ||
It is also found that, in the phylogenetic trees, novel A/H1N1 swine influenza has a much longer than expected branch relative to its nearest background clade. Furthermore, the mean genetic distance is much smaller than that background clade’s (at nucleic acid and amino acid). Based on these findings, the evolutionary history of novel swine influenza in humans is short, but has a greater history pre-human induction. Further study shows that swine flu was developed during a ten-year period. Also, this means that there is the possibility that some animal plays host to the triple reassortment of swine influenza A, and this is hypothesized to be pigs due to their ability to house the virus (though this is countered by the lack of infection of pigs and future study would be necessary to prove that pigs are able to be non-infected carriers of the disease; all subsequent assessments of swine involvement in the evolution of H1N1 in the study by Ding et al. (2009) are made based on the assumption of the ability of swine to carry the virus without infection). In the name of the scientific method, it should be recognized that an alternative hypothesis for this controversial phylogenetic tree is that this segment of evolution is slower, due to the mosaic nature of the overall process. | It is also found that, in the phylogenetic trees, novel A/H1N1 swine influenza has a much longer than expected branch relative to its nearest background clade. Furthermore, the mean genetic distance is much smaller than that background clade’s (at nucleic acid and amino acid). Based on these findings, the evolutionary history of novel swine influenza in humans is short, but has a greater history pre-human induction. Further study shows that swine flu was developed during a ten-year period. Also, this means that there is the possibility that some animal plays host to the triple reassortment of swine influenza A, and this is hypothesized to be pigs due to their ability to house the virus (though this is countered by the lack of infection of pigs and future study would be necessary to prove that pigs are able to be non-infected carriers of the disease; all subsequent assessments of swine involvement in the evolution of H1N1 in the study by Ding et al. (2009) are made based on the assumption of the ability of swine to carry the virus without infection). In the name of the scientific method, it should be recognized that an alternative hypothesis for this controversial phylogenetic tree is that this segment of evolution is slower, due to the mosaic nature of the overall process. | ||
To give a point of reference, compared to the SARS outbreak of 2003, novel H1N1 has gained the ability to transmit cross-species and from human to human. This ability is expected to be driven by strong positive selection followed by negative selection upon human host infection. The MKT test was applied to determine is this is the result. Based on the NI of the PB2, NP, and PA gene segments, PB2 and NP experience strong negative selection while PA undergoes weak positive selection. The CODEML test was employed to confirm the results of the MKT. PA was confirmed to have undergone weak positive selection. The other seven gene segments were undergoing strong purifying selection (when deleterious alleles are eliminated from a population). These results that do not concur with the hypothesis lead the experimenters to believe that the novel H1N1 swine flu is still building adaptive mutations to create positive selection (because it is still young), much like SARS did in 2003. | To give a point of reference, the study by Ding et al. compared H1N1 to the SARS outbreak of 2003, novel H1N1 has gained the ability to transmit cross-species and from human to human. This ability is expected to be driven by strong positive selection followed by negative selection upon human host infection. The MKT test was applied to determine is this is the result. Based on the NI of the PB2, NP, and PA gene segments, PB2 and NP experience strong negative selection while PA undergoes weak positive selection. The CODEML test was employed to confirm the results of the MKT. PA was confirmed to have undergone weak positive selection. The other seven gene segments were undergoing strong purifying selection (when deleterious alleles are eliminated from a population). These results that do not concur with the hypothesis lead the experimenters to believe that the novel H1N1 swine flu is still building adaptive mutations to create positive selection (because it is still young), much like SARS did in 2003. | ||
In a comparison of amino acids in novel swine influenza to its nearest clade, there is a relatively small amount of divergence in amount of amino acids than parental lineages, thus it is thought that there is fixation. There is also thought to have been a selective sweep (which thus reduced the amount of variation in the DNA sequences) early on in the evolutionary history of novel H1N1 based on the fixation of new amino acid residues. | In a comparison of amino acids in novel swine influenza to its nearest clade, there is a relatively small amount of divergence in amount of amino acids than parental lineages, thus it is thought that there is fixation. There is also thought to have been a selective sweep that results in a positive selection for a mutation which thus removes neutral variation in an allele(which thus reduced the amount of variation in the DNA sequences) early on in the evolutionary history of novel H1N1 based on the fixation (a process where two variants for an allele give way to only one single possibly phenotype) of new amino acid residues (Evolution, Futuyma). | ||
Novel swine influenza comes from the reassortment between triple-reassortant swine, Eurasia swine, and North American swine leading to the potential for new reassortment of the novel virus with H5N1 (highly pathogenic). Genetic divergence is low, and the novel H1N1 evolution outside of human hosts has a long evolutionary history. Strong purifying selection drives the induction and evolution of the virus in humans. Finally, early on in the evolutionary history of the viral strand, there was a selective sweep. | Novel swine influenza comes from the reassortment between triple-reassortant swine, Eurasia swine, and North American swine leading to the potential for new reassortment of the novel virus with H5N1 (highly pathogenic). Genetic divergence is low, and the novel H1N1 evolution outside of human hosts has a long evolutionary history. Strong purifying selection drives the induction and evolution of the virus in humans. Finally, early on in the evolutionary history of the viral strand, there was a selective sweep. | ||
| Line 41: | Line 57: | ||
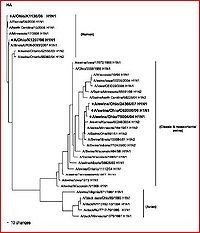
[[Image:Ha evolution.JPG|thumb|300px|right|Figure 4. The Geographic/Chronological Evolution of the individual HA gene for hemagglutinin shown on a phylogenetic tree(click to enlarge) (Zheng et al. 2009)]] | [[Image:Ha evolution.JPG|thumb|300px|right|Figure 4. The Geographic/Chronological Evolution of the individual HA gene for hemagglutinin shown on a phylogenetic tree(click to enlarge) (Zheng et al. 2009)]] | ||
Other studies and reviews have evaluated the methods and findings of Ding et al. by examining similar gene segments. A paper by Zheng et al. (2009) reviews the same eight gene segments and their abilities to characterize the evolution of novel H1N1. This paper reaffirms the techniques, methods, results, and conclusions established by Ding et al. It better explains which gene segments establish the current version of H1N1 as triple-reassortant. The HA, NA, MP, NP and NS segments were derived from swine-based influenza, PB2 and PA come from bird influenza, and finally PB1 comes from a human-hosted version of the virus. The fact that this paper confirms the efforts of other research is very encouraging to the potential eradication of the virus by way of assessing the evolutionary capabilities and the genetic regions that evolve most efficiently. | Other studies and reviews have evaluated the methods and findings of Ding et al. by examining similar gene segments. A paper by Zheng et al. (2009) reviews the same eight gene segments and their abilities to characterize the evolution of novel H1N1. This paper reaffirms the techniques, methods, results, and conclusions established by Ding et al. It better explains which gene segments establish the current version of H1N1 as triple-reassortant. The HA (see figure 4 for independent phylogeny), NA (figure 5 for individualized phylogeny), MP, NP and NS segments were derived from swine-based influenza, PB2 and PA come from bird influenza, and finally PB1 comes from a human-hosted version of the virus. The fact that this paper confirms the efforts of other research is very encouraging to the potential eradication of the virus by way of assessing the evolutionary capabilities and the genetic regions that evolve most efficiently. | ||
This paper takes another interesting explanatory step past the study done by Ding et al. (2009), depicting and discussing a group of phylogenetic trees that distinguish the evolution of the novel swine influenza A virus by geographic location (states within the United States and a few other countries). These trees isolate various versions of swine-based influenza virus strands and trace them to the location of their discoveries. This type of study has the potential to be extremely helpful to virologists and health care professionals around the world, but specifically here in the United States. Isolating individual geographic locations and origins of the disease may allow us to create area-unique vaccinations that are dependent on regions of the country. | This paper takes another interesting explanatory step past the study done by Ding et al. (2009), depicting and discussing a group of phylogenetic trees that distinguish the evolution of the novel swine influenza A virus by geographic location (states within the United States and a few other countries) and chronological time. These trees isolate various versions of swine-based influenza virus strands and trace them to the location of their discoveries. This type of study has the potential to be extremely helpful to virologists and health care professionals around the world, but specifically here in the United States. Isolating individual geographic locations and origins of the disease may allow us to create area-unique vaccinations that are dependent on regions of the country. | ||
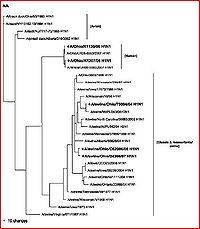
[[Image:Na evolution.JPG|thumb|300px|left|Figure 5. The geographic/chronological evolution of the NA gene for neuraminidase on a phylogenetic tree. (Click to enlarge) (Zheng et al. 2009)]]Using this data, professionals will better be able to understand the genetic nature and potential for evolution and adaptation of the virus based on the human’s environmental characteristics. This type of information is pertinent in containing, controlling, and potentially eliminating the “swine flu” pandemic. While there is already a vaccination desperately being prepared, distributed, and applied, these types of evolutionary findings are still incredibly valuable. With a complete understanding of the evolutionary history and mechanisms of swine-based influenza A, experts may begin to be able to predict the actual potential future growth and/or evolutionary potential for the virus. With proper characterization and understanding, we may be able to educationally predict and thus prevent future epidemic and pandemic situations as a result of further novel cases of H1N1 where the virus has evolved to become more dangerous to the infected human or resistant to the current methods of vaccination. Further identification of additional evolutionary and biological mechanisms of the virus will of course be necessary in this process. | [[Image:Na evolution.JPG|thumb|300px|left|Figure 5. The geographic/chronological evolution of the NA gene for neuraminidase on a phylogenetic tree. (Click to enlarge) (Zheng et al. 2009)]]Using this data, professionals will better be able to understand the genetic nature and potential for evolution and adaptation of the virus based on the human’s environmental characteristics. This type of information is pertinent in containing, controlling, and potentially eliminating the “swine flu” pandemic. While there is already a vaccination desperately being prepared, distributed, and applied, these types of evolutionary findings are still incredibly valuable. With a complete understanding of the evolutionary history and mechanisms of swine-based influenza A, experts may begin to be able to predict the actual potential future growth and/or evolutionary potential for the virus. With proper characterization and understanding, we may be able to educationally predict and thus prevent future epidemic and pandemic situations as a result of further novel cases of H1N1 where the virus has evolved to become more dangerous to the infected human or resistant to the current methods of vaccination. Further identification of additional evolutionary and biological mechanisms of the virus will of course be necessary in this process. | ||
| Line 49: | Line 65: | ||
==A Smaller Evolutionary Model: Ohio== | ==A Smaller Evolutionary Model: Ohio== | ||
[[Image:H1 evolution ohio.JPG|thumb|200px|right|Figure 6. Phylogenetic tree to demonstrate evolution of H1 influenza A types, with Ohio cases in bold. (Click to enlarge) (Yassine et al. 2009)]] | [[Image:H1 evolution ohio.JPG|thumb|200px|right|Figure 6. Phylogenetic tree to demonstrate evolution of H1 influenza A types, with Ohio cases in bold. (Click to enlarge) (Yassine et al. 2009)]] | ||
A paper by Yassine et al. (2009) puts the evolution of H1N1 on a smaller scale and examines its roots in Ohio. Laboratory tests show a genomic sequence of the virus that infected 2 humans to be exactly the same as the sequence found in three pigs the humans came into contact with at the state fair. This article was published in April, 2009. Thus, while the possibility of passing the novel influenza from swine to human has since been | A paper by Yassine et al. (2009) puts the evolution of H1N1 on a smaller scale and examines its roots in Ohio. Laboratory tests show a genomic sequence of the virus that infected 2 humans to be exactly the same as the sequence found in three pigs the humans came into contact with at the state fair. This article was published in April, 2009. Thus, while the possibility of passing the novel influenza from swine to human has since been rejected, this paper does offer us something of importance. [[Image:N1 evolution ohio.JPG|thumb|200px|left|Figure 7. Phylogenetic tree to demonstrate evolution of N1 influenza A types, with Ohio cases in bold. (Click to enlarge) (Yassine et al. 2009)]]The potential ability to isolate and pinpoint the origination of this novel virus within such a small geographic region (such as within an individual state) can prove invaluable in the theme of piecing together all the elements of the evolutionary and biological mechanisms of the newest pandemic, novel H1N1 influenza A. Figure 6 depicts the evolution of H1 influenza A types in Ohio, while figure 7 does the same for N1 influenza A types. Every small bit of information leads us to a final goal. | ||
| Line 73: | Line 89: | ||
=Mechanisms of Infection= | =Mechanisms of Infection= | ||
==Glycoproteins== | ==Glycoproteins== | ||
[[Image:Hemagglutinin interaction.jpg|thumb|300px|right|Figure 8. The interaction between hemagluttinin (red glycoprotein), neuraminidase(blue glycoprotein), and the cell membrane | [[Image:Hemagglutinin interaction.jpg|thumb|300px|right|Figure 8. The interaction between hemagluttinin (red glycoprotein), neuraminidase(blue glycoprotein), and the cell membrane which is facilitated by glycan proteins. (http://images.nigms.nih.gov/imageRepository/2505/Hemagglutinin_with_labels1.jpg)]] | ||
While it was mentioned that the hemagglutinin and neuraminidase are involved in the infection of H1N1, a more specific mechanism should be discussed to influence potential studies of antiviral methods for H1N1 influenza A. The hemagglutinin protein (HA), searches for the sialic acid receptors specifically in respiratory-lining cells’ membranes. This binding facilitates the fusion of the cell membrane and the | While it was mentioned that the hemagglutinin and neuraminidase are involved in the infection of H1N1, a more specific mechanism should be discussed to influence potential studies of antiviral methods for H1N1 influenza A. According to How H1N1 Infects Human Cells," The hemagglutinin protein (HA), searches for the sialic acid receptors specifically in respiratory-lining cells’ membranes (figure 8). This binding facilitates the fusion of the cell membrane and the virus, with the help of glycan proteins. After the fusion, the virus enters the cell. Inside the cell, the virus shell is shed and approaches the cell’s nucleus. The virus usurps the host’s replication mechanisms to make copies of itself. Important viral proteins are also synthesized at this step in the infection process. | ||
The "How H1N1 Infects Human Cells" article goes on to say that these new, replicated viral elements first leave the cell’s membrane and attempt to exit the cell and infect other cells. The sialic acid receptors on the cell’s membrane attempt to bind the HA glycoproteins which can inhibit the exit of the viral components. Here, viral evolution/mutability plays a role in expanding the capabilities of the virus. The neuraminidase glycoprotein (N) has evolved to cleave the sialic acid receptors and allow the successful exit of the viral components, in search of a new host. After infection is complete, the cell dies because the H1N1 virus triggers cell apoptosis. This is to say that the H1N1 virus can be classified as lytic, officially defined as the release of virions through the lysing of the cell to spread the virions. | |||
==The Evolution of the Glycoproteins== | ==The Evolution of the Glycoproteins== | ||
Viral evolution/mutation is a result of adapting to antibody activity. In the presence of antibodies (which are generated to inhibit re-infection by the same virus), the H protein and N protein change conformations slightly to prevent the inhibition by antibodies (Takimoto et. al 2002). This capability of the glycoproteins was established in a study prior to the H1N1 epidemic, but the general concepts of membrane fusion by these two proteins carry over from other viruses. When these two proteins were combined with 2-deoxy-2,3-dehydro-N-acetylneuraminic acid, there was a noticeable conformational change in the hydrophobic surface of a cell membrane, induced by the HA-N interaction. | Viral evolution/mutation is a result of adapting to antibody activity. In the presence of antibodies (which are generated to inhibit re-infection by the same virus), the H protein and N protein change conformations slightly (see figure 9) to prevent the inhibition by antibodies (Takimoto et. al 2002). This capability of the glycoproteins was established in a study prior to the H1N1 epidemic, but the general concepts of membrane fusion by these two proteins carry over from other viruses. When these two proteins were combined with 2-deoxy-2,3-dehydro-N-acetylneuraminic acid, there was a noticeable conformational change in the hydrophobic surface of a cell membrane, induced by the HA-N interaction. | ||
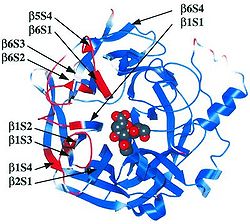
[[Image:Ha-na complex conformational change.jpg|thumb|250px|left|Figure 9. Receptor binding-induced conformational change of HN protein. A model of a monomer of the complex consisting of the Ha, N, and 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. The red regions are those regions that move more than 0.5 Å and depict a conformational change at the sialic acid-binding/hydrolysis site to the large hydrophobic surface, which is to the left of the image. (Takimoto et al. 2002)]] | |||
It is also interesting to note that influenza viruses are very capable of incorporating genetic information from other strains of influenza to increase their database of anti-antibody mechanisms. Animal host cells tend to serve as sufficient mixing vats for influenza viruses. This serves to a mechanism of the production of novel strains of influenza A ("How H1N1 Infects Human Cells"). | |||
| Line 117: | Line 130: | ||
=Conclusion= | =Conclusion= | ||
[[Image:Masked people.jpg|thumb|300px|right|Figure 10. As our knowledge increases, hopefully images like this will be prevented by vaccination and available cures/treatments. (http://dionsdailydeal.files.wordpress.com/2009/10/swine-flu.jpg)]] | |||
The growing database of information on this recently-appearing, pandemic-causing influenza A virus represents an important movement towards a better, and eventually (hopefully) a complete, understanding of the novel H1N1 influenza. The combination of the characterization of the virus and the evolutionary analyses performed provide a significant foundation for technology and intelligence to eradicate this pop-up threat to international human health. Future studies can use methods established by former studies (for example isolation and sequencing of specific gene segments and biological elements established by studies by Ding et al. (2009) and Al Farress et al.(2005) to identify the threat of evolution and adaptation of influenza viruses, including and not just limited to novel H1N1 influenza A. In doing this, vaccinations could be prepared prior to infection, thus preventing pandemic (rather than waiting until an evolved strand causes another pandemic). Also, further down the road, governmental and private scientists could use this type of data and findings to develop a cure for any of the strands of influenza that infect humans every flu season. For example, continuing address the specific roles of the hemagglutnin and neuraminidase glycoproteins may enable research professionals to develop a cure that inhibits the activity of the two pertinent proteins. The taking of big steps in characterization of the evolution of H1N1 may influence an increase in governmental spending on further studies in the field to reach the goals of proactive vaccination and possibly even cures to the seasonal influenza that plagues the world every year. While these studies and analyses represent the hard work and constant progression of an understanding in an individual setting of a topic that is important in the medical world today and is big news in the media, it also speaks to a bigger picture. It shows the ability and prospect of evolutionary studies and findings to aid governmental decisions and drive forward medical science related to development of vaccinations and cures for diseases and illnesses that have proven to be near impossible to contain up to this point. The future of the interplay between evolutionary science and microbiology to solve the medical challenges once deemed unsolvable is becoming bright. | The growing database of information on this recently-appearing, pandemic-causing influenza A virus represents an important movement towards a better, and eventually (hopefully) a complete, understanding of the novel H1N1 influenza. The combination of the characterization of the virus and the evolutionary analyses performed provide a significant foundation for technology and intelligence to eradicate this pop-up threat to international human health (preventing instances seen in figure 10). Future studies can use methods established by former studies (for example isolation and sequencing of specific gene segments and biological elements established by studies by Ding et al. (2009) and Al Farress et al.(2005) to identify the threat of evolution and adaptation of influenza viruses, including and not just limited to novel H1N1 influenza A. In doing this, vaccinations could be prepared prior to infection, thus preventing pandemic (rather than waiting until an evolved strand causes another pandemic). Also, further down the road, governmental and private scientists could use this type of data and findings to develop a cure for any of the strands of influenza that infect humans every flu season. For example, continuing address the specific roles of the hemagglutnin and neuraminidase glycoproteins may enable research professionals to develop a cure that inhibits the activity of the two pertinent proteins. The taking of big steps in characterization of the evolution of H1N1 may influence an increase in governmental spending on further studies in the field to reach the goals of proactive vaccination and possibly even cures to the seasonal influenza that plagues the world every year. While these studies and analyses represent the hard work and constant progression of an understanding in an individual setting of a topic that is important in the medical world today and is big news in the media, it also speaks to a bigger picture. It shows the ability and prospect of evolutionary studies and findings to aid governmental decisions and drive forward medical science related to development of vaccinations and cures for diseases and illnesses that have proven to be near impossible to contain up to this point. The future of the interplay between evolutionary science and microbiology to solve the medical challenges once deemed unsolvable is becoming bright. | ||
| Line 128: | Line 142: | ||
Ding, Na, Nana Wu, Qinggang Xu, Keping Chen, and Chiyu Zhang. "Molecular Evolution of Novel Swine-origina A/H1N1Influenza Among and Befor Humans." Springer Science+Business Media (2009). 20 Aug. 2009. Web. 7 Dec. 2009. | Ding, Na, Nana Wu, Qinggang Xu, Keping Chen, and Chiyu Zhang. "Molecular Evolution of Novel Swine-origina A/H1N1Influenza Among and Befor Humans." Springer Science+Business Media (2009). 20 Aug. 2009. Web. 7 Dec. 2009. | ||
Futuyma, Douglas J. Evolution. 2nd ed. Sunderland, Mass.: Sinauer Associates, 2009. Print. | |||
McKenzie, Debora. "Deadly New Flu Virus in US and Mexico May Go Pandemic." New Scientist. 24 May 2009. Web. 11 Apr. 2010. <http://www.newscientist.com/article/dn17025-deadly-new-flu-virus-in-us-and-mexico-may-go-pandemic.html>. | McKenzie, Debora. "Deadly New Flu Virus in US and Mexico May Go Pandemic." New Scientist. 24 May 2009. Web. 11 Apr. 2010. <http://www.newscientist.com/article/dn17025-deadly-new-flu-virus-in-us-and-mexico-may-go-pandemic.html>. | ||
| Line 142: | Line 158: | ||
Zheng, Kou, Hu SongNian, and Li TianXian. "Genome Evolution of Novel Influenza A (H1N1) Viruses In Humans." Science In China Press 54.13 (2009). July 2009. Web. 9 Dec. 2009. <http://journals.ohiolink.edu/ejc/pdf.cgi/Kou_Zheng.pdf?issn=10016538&issue=v54i0013&article=2159_geoniavih>. | Zheng, Kou, Hu SongNian, and Li TianXian. "Genome Evolution of Novel Influenza A (H1N1) Viruses In Humans." Science In China Press 54.13 (2009). July 2009. Web. 9 Dec. 2009. <http://journals.ohiolink.edu/ejc/pdf.cgi/Kou_Zheng.pdf?issn=10016538&issue=v54i0013&article=2159_geoniavih>. | ||
Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl10.html BIOL 238 Microbiology], 2010, [http://www.kenyon.edu/index.xml Kenyon College]. | |||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | |||
Latest revision as of 20:17, 10 August 2010
Introduction
Starting in April, 2009, a wide-spread epidemic infected the world’s population and perhaps even more so, infected the media. According to a study by Ding et al. (2009), H1N1 Influenza A virus, or better known as swine flu, made nearly 22,000 people ill and killed 125 of them by August, 2009. In all, by that time, the virus had spread through 69 countries and/or regions of the world (starting in Mexico). Ding et al. also explains that this specific strain of influenza is particularly concerning due to its uncommon ability to be passed from human to human (unlike most swine and avian A influenzas). While examining the mechanisms and characterization of the virus is important, incorporating these findings with the evolutionary capabilities and mutability of H1N1 novel influenza A represent an optimal way of examining the virus and potentially using this information to develop a cure for infected hosts.
Characterization of Influenza Viruses
General
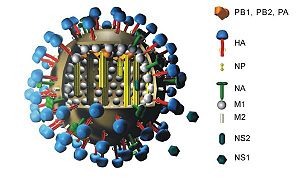
Influenza A viruses, the type that are capable of human infection, are characterized by two proteins. More specifically, the glycoproteins hemagglutinin and neuraminidase are numbered based on their identified character and are the two methods of categorization. Hemagglutinin is labeled “H” and numbered 1-16 (H16 was discovered very recently) and can be differentiated by amino acid composition (Article: "Hemagglutnin"). Its viral function is to aid in host cell binding. Specifically, it links to sialic acid located on the surface of the targeted host cell. This greatly contributes to infection capability. The other element used for subtype categorization for influenza A is the neuraminidase glycoprotein. Its role in infection is similar to that of hemagglutinin, but neuraminidase cleaves the poly-sialic surface acids in order to allow for the exiting of viral “offspring.” Neuraminidase has nine different amino acid sequences and is thus labeled N1-N9 (Al Farress et al. 2005). Figure 1 should be referenced to to observe the positioning and layout of the hemagglutinin and neuraminidase proteins on the virus. While there clearly are many combinations of the two glycoproteins that cooperate to infect a host cell, only three influenza A subtypes have been commonly found to successfully infect and transmit from human cell to human cell. This narrowing of possibilities has been crucial in fighting the biological war to control, vaccinate, and potentially someday cure the debillitating, and even sometimes fatal, virus in humans.
Hemagglutinin and Neuraminidase
A study by Al Farress et al. (2005) takes a look at the evolutionary aspect of these two glycoproteins. The study predicted that over time, based on the phylogeny of the hemagglutinin glycoprotein’s genetic origins that characterizes the type of influenza, there would be the possibility of so great a divergence between the antigenic characteristics of H1N1 and H1N2 influenza A subtypes that they could require significant changes to the vaccinations and treatments of the two strands of influenza. The implications of this conclusion and prediction by Al Faress et al.(2005) may already be felt in the form of novel H1N1 influenza A’s pandemic infection. This immediately brings to the forefront the necessity of the comprehension of the capabilities of such virus evolution and the possibilities of vaccination adaptation and thus resistance that they carry in order to prevent similar cases of influenza epidemic.
Samples of genetic regions that code for the hemagglutinin (HA 1 sequences-obtained from AH-1 strains) from three different time periods were isolated from humans experiencing influenza-like symptoms in the winters of 2001-2002, 2002-2003, and 2003-2004 in southern France. The genetic sequences were amplified by PCR. A bootstrap analysis was replicated 1000 times to perform a phylogenetic analysis. Likelihood of genetic distances was then calculated to verify the results (Figure 2).
Evolution of Swine Flu
H1N1 Is Product of Former Flu Viruses: A Study by Ding et al.
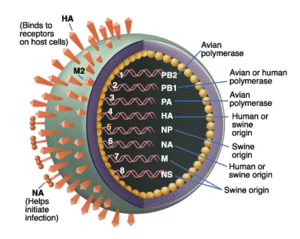
Phylogenetically, Ding et al. (2009) established that the novel swine flu has been found to originate in older viruses that were the product of reassortment of formerly triple-reassortant swine flu viruses. These older viruses were from a North American and Eurasian swine lineage that led to a lineage of avian and human roots. It has been further determined that six gene segments found in the novel swine flu virus that are close homologs to the aforementioned parent strands (polymerase PB2 (PB2), PB1, polymerase PA (PA), hemagglutinin (HA), nucleoprotein (NP), and nonstructural protein (NS)). For the evolutionary origins of these individual proteins, see figure 3. The term swine flu is derived from the fact that it has long been proven that pigs are well-known vehicles for the mixing and production of new, hybrid versions of influenza viruses. It was thought that because of this, the triple reassortment that occurs in swine is derived from avian, swine, and human and passed on to human, causing a pandemic. By the time this study was published, it was found that there were no pigs infected with the novel influenza virus and that the name and theory was unfounded. The next hypothesis, is that there has been a cross-species contamination controlled by positive selection of novel H1N1 that enables the virus to use as a human as a host and transmit from human to human. The following study by Ding et al. (2009) performs adaptive and phylogenetic analyses to test this assertion.
A few methods went came into play for testing this hypothesis. Sequences of novel A influenza derived from cases in 5 continents (excluding South American and Antarctica) and from the three different known possessing organisms: human, avian, and swine (served as a carrier, rather than an infected host). Because the HA gene in novel swine-originating flu were potentially closer homologies to A/H1N2 viruses than parent A/H1N1 viruses, A/H1N2 sequences were also obtained. For further analyses and to establish an outgroup, sequences of H5N1 and H3N2 were also retrieved. To actually perform the phylogenetic analysis, the experimenters used a program we are familiar with, CLUSTAL W (using the neighbor-joining method and 1000 replications to ensure reliability). In order to determine whether or not the novel H1N1 virus is driven by positive selection (to promote cross-species passing and human infection), the McDonald-Kreitman Test (MKT) was applied to test for natural selection of the virus. This test examines the ratio of nonsynonymous to synonymous polymorphisms in a species to detect diversity. It is compared to the neutrality index (NI), which is the ratio of nonsynonymous to synonymous fixed substitutions. It should be noted that nonsynonymous substitutions are base pair mutations where the genetic code is altered and thus the amino acid residue is changed from that of the parental sequence, and in synonymous mutations the code is preserved. Both novel H1N1 and background swine influenza A viruses were applied this test. CODEML was employed to confirm the results of MKT. This program detects selective pressure in the sequences aligned by CLUSTAL W. From these tests and methods, comparisons were made by bootstrap method. All examined viruses were divided into two clades on eight phylogenetic trees (assumes great genetic divergence in older phases of H1N1). The HA, PB2, NP, and NS gene segments were found to be a trait common with avian and swine lineages, and also three human-based lineages that were able to spread amongst humans( seasonal human A/H1N1, 1918 Spanish A/H1N1, and the novel H1N1). Thus, researchers determined that the virus is capable of evolving the ability to pass from human to human, animal to animal, and across species. Furthermore, the aforementioned human strands of the virus have differing positions of certain gene segments, presenting the feasibility of independent evolution and reassortment (which yields the potential for the human-to-human transmission capabilities of the virus) of the segments. The phylogenetic trees of the eight tested genetic segments of the three mentioned human-based traits yield some interesting data. The Na and M segments are homologous to the segments of Eurasian swine influenza lineages, while the HA segment is most associated with the North American version. These results correlate with the hypothesis of cross-species passing that resulted in elements from triple-reassortant, Eurasia, and North American swine influenza strands. Other species of swine-based influenza have shown the ability to transfer cross-species and thus it is found that the virus has developed the significant ability to reassort. Also, secondary reassortments allow for the virus to adapt and infect human hosts. This raises significant warning signs because the potential for the virus to further reassort and cause future and more lethal infection in humans exists.
It is also found that, in the phylogenetic trees, novel A/H1N1 swine influenza has a much longer than expected branch relative to its nearest background clade. Furthermore, the mean genetic distance is much smaller than that background clade’s (at nucleic acid and amino acid). Based on these findings, the evolutionary history of novel swine influenza in humans is short, but has a greater history pre-human induction. Further study shows that swine flu was developed during a ten-year period. Also, this means that there is the possibility that some animal plays host to the triple reassortment of swine influenza A, and this is hypothesized to be pigs due to their ability to house the virus (though this is countered by the lack of infection of pigs and future study would be necessary to prove that pigs are able to be non-infected carriers of the disease; all subsequent assessments of swine involvement in the evolution of H1N1 in the study by Ding et al. (2009) are made based on the assumption of the ability of swine to carry the virus without infection). In the name of the scientific method, it should be recognized that an alternative hypothesis for this controversial phylogenetic tree is that this segment of evolution is slower, due to the mosaic nature of the overall process. To give a point of reference, the study by Ding et al. compared H1N1 to the SARS outbreak of 2003, novel H1N1 has gained the ability to transmit cross-species and from human to human. This ability is expected to be driven by strong positive selection followed by negative selection upon human host infection. The MKT test was applied to determine is this is the result. Based on the NI of the PB2, NP, and PA gene segments, PB2 and NP experience strong negative selection while PA undergoes weak positive selection. The CODEML test was employed to confirm the results of the MKT. PA was confirmed to have undergone weak positive selection. The other seven gene segments were undergoing strong purifying selection (when deleterious alleles are eliminated from a population). These results that do not concur with the hypothesis lead the experimenters to believe that the novel H1N1 swine flu is still building adaptive mutations to create positive selection (because it is still young), much like SARS did in 2003.
In a comparison of amino acids in novel swine influenza to its nearest clade, there is a relatively small amount of divergence in amount of amino acids than parental lineages, thus it is thought that there is fixation. There is also thought to have been a selective sweep that results in a positive selection for a mutation which thus removes neutral variation in an allele(which thus reduced the amount of variation in the DNA sequences) early on in the evolutionary history of novel H1N1 based on the fixation (a process where two variants for an allele give way to only one single possibly phenotype) of new amino acid residues (Evolution, Futuyma).
Novel swine influenza comes from the reassortment between triple-reassortant swine, Eurasia swine, and North American swine leading to the potential for new reassortment of the novel virus with H5N1 (highly pathogenic). Genetic divergence is low, and the novel H1N1 evolution outside of human hosts has a long evolutionary history. Strong purifying selection drives the induction and evolution of the virus in humans. Finally, early on in the evolutionary history of the viral strand, there was a selective sweep.
Supplementary Evolutionary Information
Other studies and reviews have evaluated the methods and findings of Ding et al. by examining similar gene segments. A paper by Zheng et al. (2009) reviews the same eight gene segments and their abilities to characterize the evolution of novel H1N1. This paper reaffirms the techniques, methods, results, and conclusions established by Ding et al. It better explains which gene segments establish the current version of H1N1 as triple-reassortant. The HA (see figure 4 for independent phylogeny), NA (figure 5 for individualized phylogeny), MP, NP and NS segments were derived from swine-based influenza, PB2 and PA come from bird influenza, and finally PB1 comes from a human-hosted version of the virus. The fact that this paper confirms the efforts of other research is very encouraging to the potential eradication of the virus by way of assessing the evolutionary capabilities and the genetic regions that evolve most efficiently.
This paper takes another interesting explanatory step past the study done by Ding et al. (2009), depicting and discussing a group of phylogenetic trees that distinguish the evolution of the novel swine influenza A virus by geographic location (states within the United States and a few other countries) and chronological time. These trees isolate various versions of swine-based influenza virus strands and trace them to the location of their discoveries. This type of study has the potential to be extremely helpful to virologists and health care professionals around the world, but specifically here in the United States. Isolating individual geographic locations and origins of the disease may allow us to create area-unique vaccinations that are dependent on regions of the country.
Using this data, professionals will better be able to understand the genetic nature and potential for evolution and adaptation of the virus based on the human’s environmental characteristics. This type of information is pertinent in containing, controlling, and potentially eliminating the “swine flu” pandemic. While there is already a vaccination desperately being prepared, distributed, and applied, these types of evolutionary findings are still incredibly valuable. With a complete understanding of the evolutionary history and mechanisms of swine-based influenza A, experts may begin to be able to predict the actual potential future growth and/or evolutionary potential for the virus. With proper characterization and understanding, we may be able to educationally predict and thus prevent future epidemic and pandemic situations as a result of further novel cases of H1N1 where the virus has evolved to become more dangerous to the infected human or resistant to the current methods of vaccination. Further identification of additional evolutionary and biological mechanisms of the virus will of course be necessary in this process.
A Smaller Evolutionary Model: Ohio
A paper by Yassine et al. (2009) puts the evolution of H1N1 on a smaller scale and examines its roots in Ohio. Laboratory tests show a genomic sequence of the virus that infected 2 humans to be exactly the same as the sequence found in three pigs the humans came into contact with at the state fair. This article was published in April, 2009. Thus, while the possibility of passing the novel influenza from swine to human has since been rejected, this paper does offer us something of importance.
The potential ability to isolate and pinpoint the origination of this novel virus within such a small geographic region (such as within an individual state) can prove invaluable in the theme of piecing together all the elements of the evolutionary and biological mechanisms of the newest pandemic, novel H1N1 influenza A. Figure 6 depicts the evolution of H1 influenza A types in Ohio, while figure 7 does the same for N1 influenza A types. Every small bit of information leads us to a final goal.
Mechanisms of Infection
Glycoproteins
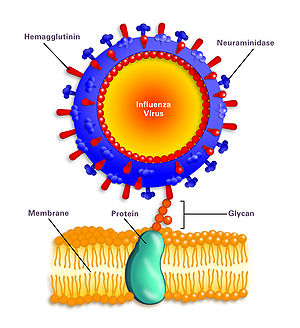
While it was mentioned that the hemagglutinin and neuraminidase are involved in the infection of H1N1, a more specific mechanism should be discussed to influence potential studies of antiviral methods for H1N1 influenza A. According to How H1N1 Infects Human Cells," The hemagglutinin protein (HA), searches for the sialic acid receptors specifically in respiratory-lining cells’ membranes (figure 8). This binding facilitates the fusion of the cell membrane and the virus, with the help of glycan proteins. After the fusion, the virus enters the cell. Inside the cell, the virus shell is shed and approaches the cell’s nucleus. The virus usurps the host’s replication mechanisms to make copies of itself. Important viral proteins are also synthesized at this step in the infection process.
The "How H1N1 Infects Human Cells" article goes on to say that these new, replicated viral elements first leave the cell’s membrane and attempt to exit the cell and infect other cells. The sialic acid receptors on the cell’s membrane attempt to bind the HA glycoproteins which can inhibit the exit of the viral components. Here, viral evolution/mutability plays a role in expanding the capabilities of the virus. The neuraminidase glycoprotein (N) has evolved to cleave the sialic acid receptors and allow the successful exit of the viral components, in search of a new host. After infection is complete, the cell dies because the H1N1 virus triggers cell apoptosis. This is to say that the H1N1 virus can be classified as lytic, officially defined as the release of virions through the lysing of the cell to spread the virions.
The Evolution of the Glycoproteins
Viral evolution/mutation is a result of adapting to antibody activity. In the presence of antibodies (which are generated to inhibit re-infection by the same virus), the H protein and N protein change conformations slightly (see figure 9) to prevent the inhibition by antibodies (Takimoto et. al 2002). This capability of the glycoproteins was established in a study prior to the H1N1 epidemic, but the general concepts of membrane fusion by these two proteins carry over from other viruses. When these two proteins were combined with 2-deoxy-2,3-dehydro-N-acetylneuraminic acid, there was a noticeable conformational change in the hydrophobic surface of a cell membrane, induced by the HA-N interaction.
It is also interesting to note that influenza viruses are very capable of incorporating genetic information from other strains of influenza to increase their database of anti-antibody mechanisms. Animal host cells tend to serve as sufficient mixing vats for influenza viruses. This serves to a mechanism of the production of novel strains of influenza A ("How H1N1 Infects Human Cells").
Conclusion

The growing database of information on this recently-appearing, pandemic-causing influenza A virus represents an important movement towards a better, and eventually (hopefully) a complete, understanding of the novel H1N1 influenza. The combination of the characterization of the virus and the evolutionary analyses performed provide a significant foundation for technology and intelligence to eradicate this pop-up threat to international human health (preventing instances seen in figure 10). Future studies can use methods established by former studies (for example isolation and sequencing of specific gene segments and biological elements established by studies by Ding et al. (2009) and Al Farress et al.(2005) to identify the threat of evolution and adaptation of influenza viruses, including and not just limited to novel H1N1 influenza A. In doing this, vaccinations could be prepared prior to infection, thus preventing pandemic (rather than waiting until an evolved strand causes another pandemic). Also, further down the road, governmental and private scientists could use this type of data and findings to develop a cure for any of the strands of influenza that infect humans every flu season. For example, continuing address the specific roles of the hemagglutnin and neuraminidase glycoproteins may enable research professionals to develop a cure that inhibits the activity of the two pertinent proteins. The taking of big steps in characterization of the evolution of H1N1 may influence an increase in governmental spending on further studies in the field to reach the goals of proactive vaccination and possibly even cures to the seasonal influenza that plagues the world every year. While these studies and analyses represent the hard work and constant progression of an understanding in an individual setting of a topic that is important in the medical world today and is big news in the media, it also speaks to a bigger picture. It shows the ability and prospect of evolutionary studies and findings to aid governmental decisions and drive forward medical science related to development of vaccinations and cures for diseases and illnesses that have proven to be near impossible to contain up to this point. The future of the interplay between evolutionary science and microbiology to solve the medical challenges once deemed unsolvable is becoming bright.
References
Al Farress, Shaker, Gaelle Cartet, Olivier Ferraris, Helene Norder, Martine Valette, and Bruno Lina. "Divergent Genetic Evolution of Hemagglutinin in Influenza A H1N1 and A H1N2 Subtypes Isolated in the South-France Since The Winter of 2001–2002." Journal of Clinical Virology 33 (2005): 230-36. Web. 10 Dec. 2009. <http://journals.ohiolink.edu/ejc/pdf.cgi/Al_Faress_Shaker.pdf?issn=13866532&issue=v33i0003&article=230_dgeohisstwo2>.
Christensen, Stephen A. "How H1N1 Infects Human Cells: Hemagglutinin and Neuraminidase Are Viral Attack Proteins." How H1N1 Infects Human Cells. Human Infections, 11 May 2009. Web. 14 Apr. 2010. <http://human-infections.suite101.com/article.cfm/how_h1n1_infects_human_cells>.
Ding, Na, Nana Wu, Qinggang Xu, Keping Chen, and Chiyu Zhang. "Molecular Evolution of Novel Swine-origina A/H1N1Influenza Among and Befor Humans." Springer Science+Business Media (2009). 20 Aug. 2009. Web. 7 Dec. 2009.
Futuyma, Douglas J. Evolution. 2nd ed. Sunderland, Mass.: Sinauer Associates, 2009. Print.
McKenzie, Debora. "Deadly New Flu Virus in US and Mexico May Go Pandemic." New Scientist. 24 May 2009. Web. 11 Apr. 2010. <http://www.newscientist.com/article/dn17025-deadly-new-flu-virus-in-us-and-mexico-may-go-pandemic.html>.
Napier, Lindsay. "The Science of Swine Flu: What Does H1N1 Mean?" Human Infections. 4 Aug. 2009. Web. 12 Apr. 2010. <http://human-infections.suite101.com/article.cfm/the_science_of_swine_flu>.
Rogers, Kara. "Hemagglutinin (glycoprotein) -- Britannica Online Encyclopedia." Hemagglutinin. Britannica. Web. 16 Apr. 2010. <http://www.britannica.com/EBchecked/topic/1093138/hemagglutinin#>.
Slonczewski, Joan L., and John W. Foster. Microbiology An Evolving Science. New York: W. W. Norton, 2008. Print.
Takimoto, Toru, Garry L. Taylor, Hellen C. Connaris, Susan J. Crennel, and Allen Portner. "Role of the Hemagglutinin-Neuraminidase Protein in the Mechanism of Paramyxovirus-Cell Membrane Fusion." Jornal of Virology. PubMed, 2002. Web. 13 Apr. 2010. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC136693/>.
Yassine, H. M., M. Khatri, Y. J. Zhang, C. W. Lee, B. A. Byrum, J. O'Quin, K. A. Smith, and Y. M. Saif. "Characterization of Triple Reassortant H1N1 Influenza A Viruses From Swine in Ohio." Veterinary Microbiology 139 (2009): 132-39. Web. 10 Dec. 2009. <http://journals.ohiolink.edu/ejc/pdf.cgi/Yassine_H.M.pdf?issn=03781135&issue=v139i1-2&article=132_cotrhiavfsio>.
Zheng, Kou, Hu SongNian, and Li TianXian. "Genome Evolution of Novel Influenza A (H1N1) Viruses In Humans." Science In China Press 54.13 (2009). July 2009. Web. 9 Dec. 2009. <http://journals.ohiolink.edu/ejc/pdf.cgi/Kou_Zheng.pdf?issn=10016538&issue=v54i0013&article=2159_geoniavih>.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2010, Kenyon College.