Hopanoid lipid: Difference between revisions
No edit summary |
|||
| Line 11: | Line 11: | ||
==Hopanoid biosynthesis== | ==Hopanoid biosynthesis== | ||
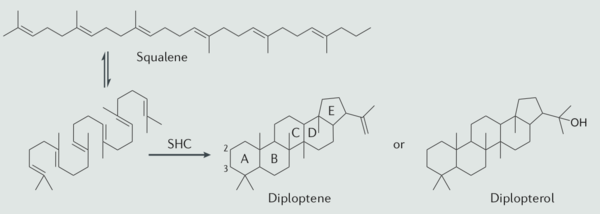
As one of the most popular natural product around the world, hopanoid, with the hopane skeleton, contains four cyclohexane and one cyclopentane with the connection of all-chair conformation. Overall, this structure will contain 30 carbon. Thus, the complete structure of hopanoid is relatively planar and rigid. The rings are methylated by the regulation of squalene precursor. The most common hopanoid compound in bacterial membrane and other structures are diplopterol and diploptene. Among around 40 variant elongated hopanoids, the most popular elongated hopanoids are aminobacteriohopanetriol and bacteriohopanetetrol in bacteria. | As one of the most popular natural product around the world, hopanoid, with the hopane skeleton, contains four cyclohexane and one cyclopentane with the connection of all-chair conformation. Overall, this structure will contain 30 carbon. Thus, the complete structure of hopanoid is relatively planar and rigid. The rings are methylated by the regulation of squalene precursor. The most common hopanoid compound in bacterial membrane and other structures are diplopterol and diploptene. Among around 40 variant elongated hopanoids, the most popular elongated hopanoids are aminobacteriohopanetriol and bacteriohopanetetrol in bacteria. | ||
The biosynthesis of hopanoid lipid contains five different stage. There are two pathway to begin the synthesis of hopanoid biosynthesis, which includes mevalonate pathway and mevalonate-independent pathway. During the first stage, the bacteria utilizes acetyl-CoA to synthesize the isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) as the C5 building blocks. Another alternative pathway is also detected in the bacterial hopanoid biosynthesis, which is the mevalonate pathway. This new-discovered biosynthesis utilized the glyceraldehyde-3-phosphate and one two-carbon produced from pyruvate decarboxylation, producing the 1-deoxyxylulose-5-phosphate, and through further arrangement the 2C-methyl-d-erythritol is produced and this chemical compound can produce IPP through more complexed reduction, phosphorylation and dehydration. The discovery of this alternative pathway biosynthesis undiscover a new way to use antibiotic, becuase protein used in this biosynthesis is specific to bacteria and scientist did not discover this bacterial enzyme in human body. Thus, new antibiotic or new medicine synthesize might trigger this specific enzyme and destroy one of the most important hopanoid lipid synthesis in bacteria. | The biosynthesis of hopanoid lipid contains five different stage. There are two pathway to begin the synthesis of hopanoid biosynthesis, which includes mevalonate pathway and mevalonate-independent pathway. During the first stage, the bacteria utilizes acetyl-CoA to synthesize the isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) as the C5 building blocks. Another alternative pathway is also detected in the bacterial hopanoid biosynthesis, which is the mevalonate pathway. This new-discovered biosynthesis utilized the glyceraldehyde-3-phosphate and one two-carbon produced from pyruvate decarboxylation, producing the 1-deoxyxylulose-5-phosphate, and through further arrangement the 2C-methyl-d-erythritol is produced and this chemical compound can produce IPP through more complexed reduction, phosphorylation and dehydration. The discovery of this alternative pathway biosynthesis undiscover a new way to use antibiotic, becuase protein used in this biosynthesis is specific to bacteria and scientist did not discover this bacterial enzyme in human body. Thus, new antibiotic or new medicine synthesize might trigger this specific enzyme and destroy one of the most important hopanoid lipid synthesis in bacteria. | ||
During the stage 2, bacterial uses two units of IPPs and with the assist of DMPP to conduct the head-to-tail elongation. This condensation will form a farnesyl diphosphate (FPP), which is also an intermediate for the biosynthesis of wither terpenes and sterols via the mevalonate pathway. During the stage 3, another condensation cause another head-to-head elongation produced from the two FPP molecules and synthesize squalene. The characteristics of the sequanlene double bond are all trans structure, producing extra stability of this sequanlene molecules. Additionally, with two similar FPP formation, the final product of stage 3 is relatively symmetrical. Also, due to their ring structure and double bond structure, the product is highly hydrophobic, increasing the functionality to their future function. | During the stage 2, bacterial uses two units of IPPs and with the assist of DMPP to conduct the head-to-tail elongation. This condensation will form a farnesyl diphosphate (FPP), which is also an intermediate for the biosynthesis of wither terpenes and sterols via the mevalonate pathway. During the stage 3, another condensation cause another head-to-head elongation produced from the two FPP molecules and synthesize squalene. The characteristics of the sequanlene double bond are all trans structure, producing extra stability of this sequanlene molecules. Additionally, with two similar FPP formation, the final product of stage 3 is relatively symmetrical. Also, due to their ring structure and double bond structure, the product is highly hydrophobic, increasing the functionality to their future function. | ||
During the stage 4, a extremely complicated synthase or isomerization reaction proceed the cyclization reaction of squalene to the hopene. As one of the most complicated one-step reaction in the biosynthesis, the long-strain squalene will first be folding into a particular shape in order to increase the accessibility of the formation of bond breaking and bond forming. During this reaction, the bacterial mechanism needs to conduct 9 stereo centers, change the connection of 13 covalent bonds and establish 5 hexane rings. This polycyclic formation and carbocation formation interest and excited lots of organic chemistry in the 20 centenary. During the last stage, the hopanoids is formated or elongated from the hopene. However, the biosynthesis of this biochemical compounds is less known and the detail of the mechanism needs further study. | |||
==References== | ==References== | ||
Revision as of 19:12, 21 April 2018
By Haofan Li
Introduction
The selectively permeable bacterial membrane plays a significantly important role in bacterial growth. Among major components of a biomembrane, lipids, though with less variable structure compared to the membrane protein, contributes to bacterial growth, fluidity as well as permeability, resistance towards stressful environments, nitrogen fixation, etc. However, until recent rapid improvement in lipid modification and measurement, many different lipid research becomes quickly evolving microbiology fields.
Hopanoid lipids are one class of well-studied modern lipid model. They are widely found on a large scale of organisms, such as bacteria, plants, and some lichens. However, no hopanoid lipids were found in archaea. Among bacteria, both gram-negative and gram-positive bacteria contains hopanoid lipids, potentially indicating the important role they play in bacterial growth and reproduction. Hopanoid lipids are pentacyclic lipids, which connected by four six-carbon rings and a five-carbon ring. With a similar structure as the four-ring eukaryotic sterols, hopanoid lipids also connect rings via sharing a carbon-carbon single bond between two ring structure, forming a stable and constant structure. Additionally, hopanoid lipids contain different hydrophobic and hydrophilic side chains, increasing the hopanoid lipid diversity and thus expanding their multiple purposes of bacteria. [1].
Hopanoid biosynthesis
As one of the most popular natural product around the world, hopanoid, with the hopane skeleton, contains four cyclohexane and one cyclopentane with the connection of all-chair conformation. Overall, this structure will contain 30 carbon. Thus, the complete structure of hopanoid is relatively planar and rigid. The rings are methylated by the regulation of squalene precursor. The most common hopanoid compound in bacterial membrane and other structures are diplopterol and diploptene. Among around 40 variant elongated hopanoids, the most popular elongated hopanoids are aminobacteriohopanetriol and bacteriohopanetetrol in bacteria.
The biosynthesis of hopanoid lipid contains five different stage. There are two pathway to begin the synthesis of hopanoid biosynthesis, which includes mevalonate pathway and mevalonate-independent pathway. During the first stage, the bacteria utilizes acetyl-CoA to synthesize the isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) as the C5 building blocks. Another alternative pathway is also detected in the bacterial hopanoid biosynthesis, which is the mevalonate pathway. This new-discovered biosynthesis utilized the glyceraldehyde-3-phosphate and one two-carbon produced from pyruvate decarboxylation, producing the 1-deoxyxylulose-5-phosphate, and through further arrangement the 2C-methyl-d-erythritol is produced and this chemical compound can produce IPP through more complexed reduction, phosphorylation and dehydration. The discovery of this alternative pathway biosynthesis undiscover a new way to use antibiotic, becuase protein used in this biosynthesis is specific to bacteria and scientist did not discover this bacterial enzyme in human body. Thus, new antibiotic or new medicine synthesize might trigger this specific enzyme and destroy one of the most important hopanoid lipid synthesis in bacteria.
During the stage 2, bacterial uses two units of IPPs and with the assist of DMPP to conduct the head-to-tail elongation. This condensation will form a farnesyl diphosphate (FPP), which is also an intermediate for the biosynthesis of wither terpenes and sterols via the mevalonate pathway. During the stage 3, another condensation cause another head-to-head elongation produced from the two FPP molecules and synthesize squalene. The characteristics of the sequanlene double bond are all trans structure, producing extra stability of this sequanlene molecules. Additionally, with two similar FPP formation, the final product of stage 3 is relatively symmetrical. Also, due to their ring structure and double bond structure, the product is highly hydrophobic, increasing the functionality to their future function.
During the stage 4, a extremely complicated synthase or isomerization reaction proceed the cyclization reaction of squalene to the hopene. As one of the most complicated one-step reaction in the biosynthesis, the long-strain squalene will first be folding into a particular shape in order to increase the accessibility of the formation of bond breaking and bond forming. During this reaction, the bacterial mechanism needs to conduct 9 stereo centers, change the connection of 13 covalent bonds and establish 5 hexane rings. This polycyclic formation and carbocation formation interest and excited lots of organic chemistry in the 20 centenary. During the last stage, the hopanoids is formated or elongated from the hopene. However, the biosynthesis of this biochemical compounds is less known and the detail of the mechanism needs further study.
References
Section
By Haofan Li

At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Sample citations: [1]
[2]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Section 1
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
Section 2
Include some current research, with at least one figure showing data.
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
