Hopanoid lipid: Difference between revisions
No edit summary |
|||
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
</b> | </b>As a major component of the selectively permeable bacterial membrane, lipids, though with a relatively monotonic structure compared to the membrane protein, plays a significant role in biochemical activity and stress tolerance. The studies of lipids are under-studied until the rapid improvement in lipid research methods, such as lipid modification, measurement, and simulation. Hopanoid lipid is one class of well-studied modern lipid model. This type of lipid is widely found on a large scale of organisms, such as bacteria, plants, and some lichens, though no hopanoid lipids are found in archaea. Both gram-negative and gram-positive bacteria contains hopanoid, which indicates the critical role they play in bacterial growth and reproduction (1). | ||
Hopanoid lipids are pentacyclic lipids, which are constituted by four six-carbon rings and one five-carbon ring at one end. With a similar structure as the four-ring eukaryotic sterols, hopanoid lipids connect rings via sharing one carbon-carbon single bond between two neighboring rings structure, forming a flat, hydrophobic, and stable chemical structure (2). Like the R group in amino acids, hopanoid lipids also contain different hydrophobic and hydrophilic side chains, increasing their diversity and expanding multiple functions in bacteria (3). From previous studies, the hopanoid lipids, as one of the essential components in a biomembrane, contributes substantially in bacterial membrane permeability and fluidity, stress resistance, nitrogen fixation and bacterial association with plants, etc. (4). <ref>[https://link.springer.com/content/pdf/10.1007%2Fs001140050592.pdf Kannenberg E. L., and Poralla K.. 1999. Hopanoid Biosynthesis and Function in Bacteria. Naturwissenschaften 86, 168–176 (1999). Springer-Verlag 1999. ]</ref>. | |||
[[Image:Tupian1.png|thumb|600px|right|The biosynthesis of hopanoid [https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-7072 CDC].]] | [[Image:Tupian1.png|thumb|600px|right|The biosynthesis of hopanoid [https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-7072 CDC].]] | ||
The hopanoid lipids play crucial roles in bacteria and bacterial interaction with animals and plants. Hopanoid lipid study, as one of the new and frontier research, remains lots of unknown to discovery. Although we understand lots of broad interaction between the hopanoid lipids and other macromolecules, the detailed mechanisms remain unascertained. Overall, future studies are needed to unveil more interesting facts of hopanoid lipids. | |||
==Hopanoid biosynthesis== | ==Hopanoid biosynthesis== | ||
One of the universal natural products, hopanoid lipids, with a basic hopane skeleton structure, contains four cyclohexane and one cyclopentane, all of which connecting with each other by sharing one carbon-carbon single bond. Thus, those carbons rings, with the all-chair conformation, form a planar, hydrophobic, and stable structure with thirty carbons. Based on the skeleton structure, the hopanoid lipids are further modified by the methylation of the squalene precursor. The most common simple hopanoid compounds in the bacterial membrane are diplopterol and diploptene. Among the 40 widely-found elongated hopanoids in bacterial membrane, the most popular hopanoids are aminobacterialhexanetriol and bacteriohopanetetrol. | |||
Around ten percents of bacteria show the ability to auto-synthesize hopanoid lipids, such as Cyanobacteria and Bacilli (4). The biosynthesis of hopanoid lipids is generally divided into five different stages. In stage one, there are two pathways to begin the synthesis of hopanoid, which include the mevalonate pathway and the mevalonate-independent pathway. In the mevalonate pathway, bacteria utilize acetyl-CoA produced during bacterial respiration to synthesize the dimethylallyl pyrophosphate and isopentenyl pyrophosphate (12). Both products from this pathway are used as the five-carbon building blocks. The mevalonate-independent pathway utilizes the glyceraldehyde-3-phosphate and two carbon compounds to produce dimethylallyl pyrophosphate through a series of complicated reaction, such as pyruvate decarboxylation, phosphorylation, and dehydration. The mevalonate-independent pathway, a biosynthesis commonly found in plant plastid organelles, is also found in some wide-known pathogens, such as Mycobacterium tuberculosis (5). This discovery excites the antibiotic treatment towards pathogens because this alternative pathway indicates a potential antibiotic target for bacterial inhibition. Fosmidomycin, isolated from the secondary metabolism of Streptomyces, a genus of Gram-positive soil bacteria, inhibits the activity of 1-deoxy-D-xylulose 5-phosphate (DXP) reductoisomerase, one essential enzyme utilized in the mevalonate-independent pathway. This enzyme is not produced in the human body, and thus this potential new antibiotic candidate might destroy one of the crucial bacterial enzymes without negatively influence human body (6, 11). | |||
The stage two and three are similar to the sterols synthesis mechanisms. In stage two, bacteria, with the assist of isopentenyl pyrophosphate, connects two units of dimethylallyl pyrophosphate via head-to-tail condensation, forming one unit of farnesyl diphosphate. In stage three, two units of the product from stage two are reductively connected through head-to-head condensation with the assist of synthesizing squalene. Most of the final products of stage three show trans conformations with high stability. Additionally, due to the condensation of two similar farnesyl diphosphate molecule, this thirty-carbon compound shows a symmetrical characteristic. | |||
In stage four, the squalene proceeds the cyclization reaction to produce hopene. The organic reaction happening in stage four is one of the most complicated one-step organic biosynthesis. The squalene first folds into a proper orientation, therefore increasing the kinetics of this reaction and proceeding the polycyclic formation. This cyclization reaction includes the modification of nine stereocenters, the connection change of thirteen covalent bonds, and the establishment of four cyclohexane and one cyclopentane. The discovery of this one-step complicated carbocation formation reaction interested twenty-century organic chemists. In stage five, bacteria modify the unfinished product from stage four via side chain formation and core backbone structure modification. In the side chain formation, various enzyme, especially those coded by the hpn genes, further diversify the structure of hopanoid lipids via the addition of a different type of side chain. The methylation of the core structure happens in the backbone structure modification. A few organisms also conduct the desaturation of the five rings. Less biochemical mechanisms are known for the stage five, and the details of this process need further studies (4). | |||
==Bacterial membrane permeability and fluidity== | |||
The hopanoid lipid, which contains a majority hydrophobic five-ring structure with either polar or nonpolar side chains, shows hydrophobic and relatively stable characteristics. Thus, the majority of the hopanoid lipid can travel and intercalate into the hydrophobic layer of the double membrane and interact with the hydrophobic lipid tail. In other words, the hydrophobic hopanoid lipid inserts between the nonpolar fatty acid, decreasing the extra space between the lipid hydrophobic tail. Therefore, the movement of the lipid tail reduces, increasing the membrane thickness and decreasing its fluidity. | |||
Various side chains of hopanoid lipids might have different influences on the bacterial membrane characteristic and properties. The variance in side chain changes the function of hopanoid lipid substantially. For example, the 3-methyl-BHT, with a large hydrophilic side chain, experiences difficulties in inserting totally into the membrane. However, the diploptene with a hydrophobic side chain relatively freely moves inside the lipid tails. Therefore, hopanoid lipids with unique functional groups affect its distribution inside the bacterial membrane, the way that hopanoid lipid interacts with the environment, and hopanoid lipids interaction with other hopanoid lipids’ function groups. In the membrane dynamic simulation, the amphiphilic hopanoid lipids, with a hydrophobic skeleton and hydrophilic side chain, show an upright orientation, which is similar to other phospholipids. Due to their unique orientation, they can interact with other lipid layers, and thus condense the overall structure and increase the integration of the bacterial membrane. On the other hand, some hydrophobic hopanoid lipids with hydrophobic side chain show a strong ability to transferring into the nonpolar part of the biomembrane, which increases the lipids thickness by limiting the movement of the hydrophobic tails. | |||
The sterol in eukaryotic and hopanoid lipids in prokaryotes show similarities in functions of membrane stability, but they might contribute to the stability differently. In one dynamic lipid simulation, researchers use cholesterol, one kind of sterol, and diplopterol, one type of hopanoid lipids, to analyze their ability in membrane organization. Both cholesterol and diplopterol prohibit the formation of gel phase during membrane simulation, and they also order the orientation of N-stearoyl-D-erythro-sphingosylphosphorylcholine, one artificial sphingolipid (7). However, the cholesterol induces the immiscible formation of both liquid disorder formation and liquid ordered configuration, while the diplopterol shows a separation of those two lipid formations. Thus, those two different configurations of lipids indicate the various mechanisms that sterol and hopanoid lipids use within the cell membrane. Additionally, the influence of either sterol or hopanoid lipids might be different in vivo. In the artificial membrane, the membrane with the interaction of 2-methyl-diplopterol, one methylated hopanoid lipid, shows higher thickness compared that of cholesterol. Thus, the addition of 2-methyl-diplopterol in biomembrane decreases the fluidity and thus increases the stability. However, the membrane of Rhodopseudomonas palustris strain with a hpnP mutation, one gene that codes for the production of 2-methyl-diplopterol, shows no significant differences in the thickness of bacterial membrane compared with that of wild-type with a functional hpnP gene. Therefore, the in vivo condition is more complexed compared to the lipid dynamic simulation. | |||
==Stress tolerance== | |||
The abundance and diversity of hopanoid lipids positively influence bacterial resistance to environmental stress, such as the extreme pH, high pressure, non-optical temperature, and high concentration of antibiotic or other lethal chemical compounds. Although various bacteria show that hopanoid lipids have different resistance to the different stress environments, researchers do find that under the extreme condition, the biosynthesis of hopanoid lipids increases in most bacterial strains making them have an increased resistance to the surrounding. When Rhodopseudomonas palustris is placed in an extreme environment, such as high temperature or low pH condition, the stress induces the activation of the ecfG gene, which is a general stress regulator for the alphaproteobacteria. The response factors and regulators synthesized by ecfG upregulate the expression of hpnP, a gene that codes for the hopanoid methylase. Therefore, the activation of hpnP increases the methylation of hopanoid lipids in bacteria, perhaps providing them with methylation on hopanoid lipids. Those methylation increases the hydrophobic character of hopanoid lipid and would further stabilize the hydrophobic tail of phospholipids, which might increase the membrane resistance to the potential danger (10). | |||
Different hopanoid lipids related mutations show different stress tolerances. Bile salts show no negative influence on the majority of the Gram-negative bacteria because those molecules are not able to transfer across the outer membrane of the Gram-negative bacteria. Mutated bacteria, without the protection of hopanoid lipids, are vulnerable to the high bile salt conditions. However, different mutations in the same bacteria strain show different levels of bile salts sensitivity. The R. palustris with the shc mutation, which is essential for the production of all type of hopanoid lipids, shows no bile salts tolerance. At the same time, the hpnH mutant, with one deficient gene which codes for the production of diplotene and diplopterol, shows resistance towards the bile salts but performs a slower growth rate compared to that of the wild-type. The dpnO mutant, with a non-functional code, need to produce one essential bacterial hopanoid lipid, aminobacteriohopanetriol, shows no influence on bacterial growth and culture density in the high bile salts compared to the wild-type strain (13). | |||
The hopanoid lipids might also assist the diverse functions of the bacterial membrane. One methylobacterium extorquens strain, with a non-functional hopanoid lipid production, shows an inefficient antibiotic resistance. This strain cannot pump out the antibiotics due to its non-functional multidrug efflux. Thus, the hopanoid lipid might contribute and assist the function of membrane proteins. The collaboration of the hopanoid lipid and membrane protein enhance the antibiotic resistance and help bacteria to maintain the homeostasis between the cytoplasm and extracellular environment. Additionally, bacterial membrane, one of the most significant energy production places, requires the presence of effective hopanoid lipids to achieve energy production and storage. Nostoc punctiforme, with a hopanoid lipid mutation, show a decrease in energy storage compared to that of the wild-type strain. | |||
The stress tolerance induced by hopanoid lipid varies among different stress conditions. The hpnP codes for the production of 2-mehopanoid. Some bacteria with this mutation, such as Rhodopseudomonas palustris and Nostoc punctiforme, shows a lower resistance to the low pH conditions. However, the increasing production of 2-mehopanoid only assist the resistance towards the high proton concentration but does not contribute to any other resistances towards, such as the high temperature and high antibiotic concentration. | |||
The function of hopanoid is more significant in the stress-resistance specified cell. The filament of Nostoc punctiforme, one kind of filamentous cyanobacteria, are constituted by the vegetative cells. That specified cell conducts photosynthesis under the environment with abundant nutrients. Therefore, they fix the carbon dioxide in the environment and shows a high growth and reproduction rate. In this photosynthetic cells, the hopanoid concentration shows negligible influence on the stress response. However, under the environment with poor nutrient concentration, the Nostoc punctiforme develop the akinete cells through differentiation, which contain higher resistance toward the cold and dry environment. The akinete cell, keeping their stable structure and bacterial activity, stay in low energy consumption and store energy for many years until they encounter an environment with rich resources. In the akinete cell, a high concentration of hopanoid lipids are detected in the bacterial membrane. Previous studies found that the mutation without functional hopanoid lipid shows less resistance to the extracellular resistance. Similarly, Streptomyces coelicolor, one gram-positive soil bacterium, forms vegetative cells under nutrient-rich environment. These vegetative cells have a low hopanoid lipid concentration. However, at the end of their life cycle, they produce and accumulate hopanoid lipids and form spore via sporulation, which can assist their resistance under the extreme condition. | |||
The detailed mechanism of stress tolerance induced by the hopanoid lipids remains unclear and requires further studies. One possible hypothesis is that the presence of hopanoid lipids decreases the fluidity and permeability of the bacterial membrane. The high concentration of hopanoid increases the integrity of the membrane. Those lipid molecules form the stronger interaction with the membrane lipid, and the side chains of the hopanoid lipids might form polar attractions with other molecules. Overall, the biomembrane becomes more stable, and the leakage of lethal molecules decrease. Therefore, under the low pH or high antibiotic condition, the proton and antibiotic compounds might experience difficulty across the bacterial membrane due to decreased permeability. Therefore, bacteria show higher resistance towards the high concentration of proton, antibiotics, and other lethal molecules. Similarly, the orientation and the distribution of hopanoid lipids also contribute to the heat resistance of bacteria. Bacteria, with a thicker membrane, obtains heat protection and keep the homeostasis of their cytoplasm. As the environmental temperature increases, the hopanoid lipids tend to move to the place between two leaflets. In the lipid dynamic simulation at room temperature (298 K), most of the diplopterol molecules gather in the area between the head and the tail of phospholipid. The distribution of hopanoid lipids shows a large separation. However, as the temperature increases, the distribution of diplopterol molecules migrate to the empty plates between the tail of two leaflets, largely increasing the integration and thickness of the bacterial membrane, creating a substantial protection for the thermophiles (9). | |||
==Nitrogen fixation== | |||
Various bacteria, with the ability of hopanoid production, can fix nitrogen. Bacteria, such as Beijerinckia, Frankia, Anabaena, Burkholderia, etc., show a positive correlation between the hopanoid production and nitrogen fixation. The majority of nitrogen-fixing bacteria live in close physical correlation with plants, such as alfalfa, soil beans, peas, etc. Both bacteria and plants can take advantage of this relationship. Bradyrhizobium spp. derive energy and other important chemical cofactors from host plants. The plants also can protect them from the stress in the environmental, such as competition with another microbiome, high proton concentration, etc. Equally, plants benefit from the nitrogen oxides produced by the nitrogen-fixing bacteria, and therefore plants show an enhanced growth and outcompete other competitors. There are also free-living nitrogen-fixing microbes, such as Anabaena spp., Frankia spp., etc. However, most of those bacteria do not proceed nitrogen fixation without coexisting with plants. | |||
The legume-rhizobia root nodule symbiosis is among one of the most widely studied plants and bacteria associations. The production of hopanoid lipids influences the bacterial symbiosis with the host plant. The Bradyrhizobium spp., with shc mutation, produce the negligible amount of hopanoid lipids. Therefore, this bacteria strain is incapable of integrating with plants. Additionally, different types of hopanoid lipid contribute differently to the bacterial association with plants. The Bradyrhizobium diazoefficiens with a hpnP mutation cannot produce the extended hopanoids. Their association with the Aeschynomene afraspera results in a morphologically disorganized nodule, because the A. afraspera shows nitrogen starvation, indicating a decreased nitrogen-fixing ability of the hpnP mutant. At the same time, the B. diazoefficiens with a hpnP mutation shows no production of 2-mehopanoids. The nitrogen fixation of this mutation strain, in contrast, show no differences compared to that of the wild-type strain (14). | |||
The presence of the hopanoid lipid might also contribute to the competition between bacteria to inhabit a host. For example, the Bradyrhizobium and Burkholderia, both of which contain a high concentration of hopanoid lipids, show high resistance to high temperature and low pH condition. Thus, in consideration of global warming and increasing soil acidity, both of those bacteria might outcompete other bacteria in soil and plant. The mutation strain shc, which produces no hopanoid lipids shows an inability in long-term colonization of a plant. Previous studies have found that in the beginning, this bacteria grow and develop in host normally. However, after a few days, this bacteria starts to degenerate and be recycled by their host. | |||
Bacteria conduct the majority of nitrogen fixation in the low-oxygen concentration environment. Nitrogenase, the core enzyme in nitrogen fixation, is sensitive and vulnerable in the presence of oxygen. Thus, the majority of the nitrogen-fixing bacteria exist under the anaerobic environment. Sometimes, nitrogen fixation can also be conducted at high oxygen concentration. Under this condition, bacteria develop more sophisticated mechanisms to protect the nitrogenase and achieve nitrogen fixation. The free-living nitrogen-fixing bacteria, Azotobacter vinelandii, develop a various way to fix nitrogen under high oxygen condition. Previous studies observed that the activity of nitrogenase activity has negligible decrease under the air saturation from 30-100%. The Azotobacter vinelandii decrease their cellular surface area. Therefore, for each unit of cytoplasm, there is larger protection from the bacterial membrane. Also, under high oxygen condition, they also increase their oxygen consumption via a high respiration rate, thus creating an optional anaerobic environment for nitrogen fixation. The A. vinelandii also synthesize alginate to create alginate capsule, a thick barrier which limits the oxygen fission and protect the nitrogenase from the high oxygen concentrated environment. Under the lower oxygen concentrated environment, A. vinelandii develops a loose alginate capsule, while under the high concentration, they build up a compact and thick alginate capsule. Additionally, when the bacteria interacts with or inhabit plants, the host plants synthesize leghemoglobin molecules, one type of oxygen-transport metalloproteins. This hemoglobin scavenges oxygen from the root nodules of the leguminous plants, creating an optimal environment for nitrogenase activity (8). | |||
==Conclusion== | ==Conclusion== | ||
Revision as of 03:12, 24 April 2018
By Haofan Li
Introduction
As a major component of the selectively permeable bacterial membrane, lipids, though with a relatively monotonic structure compared to the membrane protein, plays a significant role in biochemical activity and stress tolerance. The studies of lipids are under-studied until the rapid improvement in lipid research methods, such as lipid modification, measurement, and simulation. Hopanoid lipid is one class of well-studied modern lipid model. This type of lipid is widely found on a large scale of organisms, such as bacteria, plants, and some lichens, though no hopanoid lipids are found in archaea. Both gram-negative and gram-positive bacteria contains hopanoid, which indicates the critical role they play in bacterial growth and reproduction (1).
Hopanoid lipids are pentacyclic lipids, which are constituted by four six-carbon rings and one five-carbon ring at one end. With a similar structure as the four-ring eukaryotic sterols, hopanoid lipids connect rings via sharing one carbon-carbon single bond between two neighboring rings structure, forming a flat, hydrophobic, and stable chemical structure (2). Like the R group in amino acids, hopanoid lipids also contain different hydrophobic and hydrophilic side chains, increasing their diversity and expanding multiple functions in bacteria (3). From previous studies, the hopanoid lipids, as one of the essential components in a biomembrane, contributes substantially in bacterial membrane permeability and fluidity, stress resistance, nitrogen fixation and bacterial association with plants, etc. (4). [1].
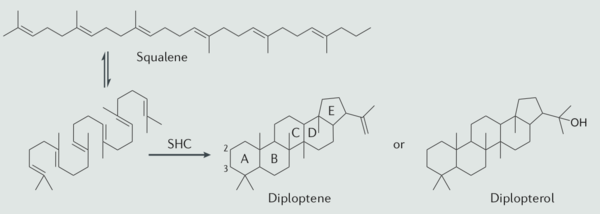
The hopanoid lipids play crucial roles in bacteria and bacterial interaction with animals and plants. Hopanoid lipid study, as one of the new and frontier research, remains lots of unknown to discovery. Although we understand lots of broad interaction between the hopanoid lipids and other macromolecules, the detailed mechanisms remain unascertained. Overall, future studies are needed to unveil more interesting facts of hopanoid lipids.
Hopanoid biosynthesis
One of the universal natural products, hopanoid lipids, with a basic hopane skeleton structure, contains four cyclohexane and one cyclopentane, all of which connecting with each other by sharing one carbon-carbon single bond. Thus, those carbons rings, with the all-chair conformation, form a planar, hydrophobic, and stable structure with thirty carbons. Based on the skeleton structure, the hopanoid lipids are further modified by the methylation of the squalene precursor. The most common simple hopanoid compounds in the bacterial membrane are diplopterol and diploptene. Among the 40 widely-found elongated hopanoids in bacterial membrane, the most popular hopanoids are aminobacterialhexanetriol and bacteriohopanetetrol.
Around ten percents of bacteria show the ability to auto-synthesize hopanoid lipids, such as Cyanobacteria and Bacilli (4). The biosynthesis of hopanoid lipids is generally divided into five different stages. In stage one, there are two pathways to begin the synthesis of hopanoid, which include the mevalonate pathway and the mevalonate-independent pathway. In the mevalonate pathway, bacteria utilize acetyl-CoA produced during bacterial respiration to synthesize the dimethylallyl pyrophosphate and isopentenyl pyrophosphate (12). Both products from this pathway are used as the five-carbon building blocks. The mevalonate-independent pathway utilizes the glyceraldehyde-3-phosphate and two carbon compounds to produce dimethylallyl pyrophosphate through a series of complicated reaction, such as pyruvate decarboxylation, phosphorylation, and dehydration. The mevalonate-independent pathway, a biosynthesis commonly found in plant plastid organelles, is also found in some wide-known pathogens, such as Mycobacterium tuberculosis (5). This discovery excites the antibiotic treatment towards pathogens because this alternative pathway indicates a potential antibiotic target for bacterial inhibition. Fosmidomycin, isolated from the secondary metabolism of Streptomyces, a genus of Gram-positive soil bacteria, inhibits the activity of 1-deoxy-D-xylulose 5-phosphate (DXP) reductoisomerase, one essential enzyme utilized in the mevalonate-independent pathway. This enzyme is not produced in the human body, and thus this potential new antibiotic candidate might destroy one of the crucial bacterial enzymes without negatively influence human body (6, 11).
The stage two and three are similar to the sterols synthesis mechanisms. In stage two, bacteria, with the assist of isopentenyl pyrophosphate, connects two units of dimethylallyl pyrophosphate via head-to-tail condensation, forming one unit of farnesyl diphosphate. In stage three, two units of the product from stage two are reductively connected through head-to-head condensation with the assist of synthesizing squalene. Most of the final products of stage three show trans conformations with high stability. Additionally, due to the condensation of two similar farnesyl diphosphate molecule, this thirty-carbon compound shows a symmetrical characteristic.
In stage four, the squalene proceeds the cyclization reaction to produce hopene. The organic reaction happening in stage four is one of the most complicated one-step organic biosynthesis. The squalene first folds into a proper orientation, therefore increasing the kinetics of this reaction and proceeding the polycyclic formation. This cyclization reaction includes the modification of nine stereocenters, the connection change of thirteen covalent bonds, and the establishment of four cyclohexane and one cyclopentane. The discovery of this one-step complicated carbocation formation reaction interested twenty-century organic chemists. In stage five, bacteria modify the unfinished product from stage four via side chain formation and core backbone structure modification. In the side chain formation, various enzyme, especially those coded by the hpn genes, further diversify the structure of hopanoid lipids via the addition of a different type of side chain. The methylation of the core structure happens in the backbone structure modification. A few organisms also conduct the desaturation of the five rings. Less biochemical mechanisms are known for the stage five, and the details of this process need further studies (4).
Bacterial membrane permeability and fluidity
The hopanoid lipid, which contains a majority hydrophobic five-ring structure with either polar or nonpolar side chains, shows hydrophobic and relatively stable characteristics. Thus, the majority of the hopanoid lipid can travel and intercalate into the hydrophobic layer of the double membrane and interact with the hydrophobic lipid tail. In other words, the hydrophobic hopanoid lipid inserts between the nonpolar fatty acid, decreasing the extra space between the lipid hydrophobic tail. Therefore, the movement of the lipid tail reduces, increasing the membrane thickness and decreasing its fluidity.
Various side chains of hopanoid lipids might have different influences on the bacterial membrane characteristic and properties. The variance in side chain changes the function of hopanoid lipid substantially. For example, the 3-methyl-BHT, with a large hydrophilic side chain, experiences difficulties in inserting totally into the membrane. However, the diploptene with a hydrophobic side chain relatively freely moves inside the lipid tails. Therefore, hopanoid lipids with unique functional groups affect its distribution inside the bacterial membrane, the way that hopanoid lipid interacts with the environment, and hopanoid lipids interaction with other hopanoid lipids’ function groups. In the membrane dynamic simulation, the amphiphilic hopanoid lipids, with a hydrophobic skeleton and hydrophilic side chain, show an upright orientation, which is similar to other phospholipids. Due to their unique orientation, they can interact with other lipid layers, and thus condense the overall structure and increase the integration of the bacterial membrane. On the other hand, some hydrophobic hopanoid lipids with hydrophobic side chain show a strong ability to transferring into the nonpolar part of the biomembrane, which increases the lipids thickness by limiting the movement of the hydrophobic tails.
The sterol in eukaryotic and hopanoid lipids in prokaryotes show similarities in functions of membrane stability, but they might contribute to the stability differently. In one dynamic lipid simulation, researchers use cholesterol, one kind of sterol, and diplopterol, one type of hopanoid lipids, to analyze their ability in membrane organization. Both cholesterol and diplopterol prohibit the formation of gel phase during membrane simulation, and they also order the orientation of N-stearoyl-D-erythro-sphingosylphosphorylcholine, one artificial sphingolipid (7). However, the cholesterol induces the immiscible formation of both liquid disorder formation and liquid ordered configuration, while the diplopterol shows a separation of those two lipid formations. Thus, those two different configurations of lipids indicate the various mechanisms that sterol and hopanoid lipids use within the cell membrane. Additionally, the influence of either sterol or hopanoid lipids might be different in vivo. In the artificial membrane, the membrane with the interaction of 2-methyl-diplopterol, one methylated hopanoid lipid, shows higher thickness compared that of cholesterol. Thus, the addition of 2-methyl-diplopterol in biomembrane decreases the fluidity and thus increases the stability. However, the membrane of Rhodopseudomonas palustris strain with a hpnP mutation, one gene that codes for the production of 2-methyl-diplopterol, shows no significant differences in the thickness of bacterial membrane compared with that of wild-type with a functional hpnP gene. Therefore, the in vivo condition is more complexed compared to the lipid dynamic simulation.
Stress tolerance
The abundance and diversity of hopanoid lipids positively influence bacterial resistance to environmental stress, such as the extreme pH, high pressure, non-optical temperature, and high concentration of antibiotic or other lethal chemical compounds. Although various bacteria show that hopanoid lipids have different resistance to the different stress environments, researchers do find that under the extreme condition, the biosynthesis of hopanoid lipids increases in most bacterial strains making them have an increased resistance to the surrounding. When Rhodopseudomonas palustris is placed in an extreme environment, such as high temperature or low pH condition, the stress induces the activation of the ecfG gene, which is a general stress regulator for the alphaproteobacteria. The response factors and regulators synthesized by ecfG upregulate the expression of hpnP, a gene that codes for the hopanoid methylase. Therefore, the activation of hpnP increases the methylation of hopanoid lipids in bacteria, perhaps providing them with methylation on hopanoid lipids. Those methylation increases the hydrophobic character of hopanoid lipid and would further stabilize the hydrophobic tail of phospholipids, which might increase the membrane resistance to the potential danger (10).
Different hopanoid lipids related mutations show different stress tolerances. Bile salts show no negative influence on the majority of the Gram-negative bacteria because those molecules are not able to transfer across the outer membrane of the Gram-negative bacteria. Mutated bacteria, without the protection of hopanoid lipids, are vulnerable to the high bile salt conditions. However, different mutations in the same bacteria strain show different levels of bile salts sensitivity. The R. palustris with the shc mutation, which is essential for the production of all type of hopanoid lipids, shows no bile salts tolerance. At the same time, the hpnH mutant, with one deficient gene which codes for the production of diplotene and diplopterol, shows resistance towards the bile salts but performs a slower growth rate compared to that of the wild-type. The dpnO mutant, with a non-functional code, need to produce one essential bacterial hopanoid lipid, aminobacteriohopanetriol, shows no influence on bacterial growth and culture density in the high bile salts compared to the wild-type strain (13).
The hopanoid lipids might also assist the diverse functions of the bacterial membrane. One methylobacterium extorquens strain, with a non-functional hopanoid lipid production, shows an inefficient antibiotic resistance. This strain cannot pump out the antibiotics due to its non-functional multidrug efflux. Thus, the hopanoid lipid might contribute and assist the function of membrane proteins. The collaboration of the hopanoid lipid and membrane protein enhance the antibiotic resistance and help bacteria to maintain the homeostasis between the cytoplasm and extracellular environment. Additionally, bacterial membrane, one of the most significant energy production places, requires the presence of effective hopanoid lipids to achieve energy production and storage. Nostoc punctiforme, with a hopanoid lipid mutation, show a decrease in energy storage compared to that of the wild-type strain.
The stress tolerance induced by hopanoid lipid varies among different stress conditions. The hpnP codes for the production of 2-mehopanoid. Some bacteria with this mutation, such as Rhodopseudomonas palustris and Nostoc punctiforme, shows a lower resistance to the low pH conditions. However, the increasing production of 2-mehopanoid only assist the resistance towards the high proton concentration but does not contribute to any other resistances towards, such as the high temperature and high antibiotic concentration.
The function of hopanoid is more significant in the stress-resistance specified cell. The filament of Nostoc punctiforme, one kind of filamentous cyanobacteria, are constituted by the vegetative cells. That specified cell conducts photosynthesis under the environment with abundant nutrients. Therefore, they fix the carbon dioxide in the environment and shows a high growth and reproduction rate. In this photosynthetic cells, the hopanoid concentration shows negligible influence on the stress response. However, under the environment with poor nutrient concentration, the Nostoc punctiforme develop the akinete cells through differentiation, which contain higher resistance toward the cold and dry environment. The akinete cell, keeping their stable structure and bacterial activity, stay in low energy consumption and store energy for many years until they encounter an environment with rich resources. In the akinete cell, a high concentration of hopanoid lipids are detected in the bacterial membrane. Previous studies found that the mutation without functional hopanoid lipid shows less resistance to the extracellular resistance. Similarly, Streptomyces coelicolor, one gram-positive soil bacterium, forms vegetative cells under nutrient-rich environment. These vegetative cells have a low hopanoid lipid concentration. However, at the end of their life cycle, they produce and accumulate hopanoid lipids and form spore via sporulation, which can assist their resistance under the extreme condition.
The detailed mechanism of stress tolerance induced by the hopanoid lipids remains unclear and requires further studies. One possible hypothesis is that the presence of hopanoid lipids decreases the fluidity and permeability of the bacterial membrane. The high concentration of hopanoid increases the integrity of the membrane. Those lipid molecules form the stronger interaction with the membrane lipid, and the side chains of the hopanoid lipids might form polar attractions with other molecules. Overall, the biomembrane becomes more stable, and the leakage of lethal molecules decrease. Therefore, under the low pH or high antibiotic condition, the proton and antibiotic compounds might experience difficulty across the bacterial membrane due to decreased permeability. Therefore, bacteria show higher resistance towards the high concentration of proton, antibiotics, and other lethal molecules. Similarly, the orientation and the distribution of hopanoid lipids also contribute to the heat resistance of bacteria. Bacteria, with a thicker membrane, obtains heat protection and keep the homeostasis of their cytoplasm. As the environmental temperature increases, the hopanoid lipids tend to move to the place between two leaflets. In the lipid dynamic simulation at room temperature (298 K), most of the diplopterol molecules gather in the area between the head and the tail of phospholipid. The distribution of hopanoid lipids shows a large separation. However, as the temperature increases, the distribution of diplopterol molecules migrate to the empty plates between the tail of two leaflets, largely increasing the integration and thickness of the bacterial membrane, creating a substantial protection for the thermophiles (9).
Nitrogen fixation
Various bacteria, with the ability of hopanoid production, can fix nitrogen. Bacteria, such as Beijerinckia, Frankia, Anabaena, Burkholderia, etc., show a positive correlation between the hopanoid production and nitrogen fixation. The majority of nitrogen-fixing bacteria live in close physical correlation with plants, such as alfalfa, soil beans, peas, etc. Both bacteria and plants can take advantage of this relationship. Bradyrhizobium spp. derive energy and other important chemical cofactors from host plants. The plants also can protect them from the stress in the environmental, such as competition with another microbiome, high proton concentration, etc. Equally, plants benefit from the nitrogen oxides produced by the nitrogen-fixing bacteria, and therefore plants show an enhanced growth and outcompete other competitors. There are also free-living nitrogen-fixing microbes, such as Anabaena spp., Frankia spp., etc. However, most of those bacteria do not proceed nitrogen fixation without coexisting with plants.
The legume-rhizobia root nodule symbiosis is among one of the most widely studied plants and bacteria associations. The production of hopanoid lipids influences the bacterial symbiosis with the host plant. The Bradyrhizobium spp., with shc mutation, produce the negligible amount of hopanoid lipids. Therefore, this bacteria strain is incapable of integrating with plants. Additionally, different types of hopanoid lipid contribute differently to the bacterial association with plants. The Bradyrhizobium diazoefficiens with a hpnP mutation cannot produce the extended hopanoids. Their association with the Aeschynomene afraspera results in a morphologically disorganized nodule, because the A. afraspera shows nitrogen starvation, indicating a decreased nitrogen-fixing ability of the hpnP mutant. At the same time, the B. diazoefficiens with a hpnP mutation shows no production of 2-mehopanoids. The nitrogen fixation of this mutation strain, in contrast, show no differences compared to that of the wild-type strain (14).
The presence of the hopanoid lipid might also contribute to the competition between bacteria to inhabit a host. For example, the Bradyrhizobium and Burkholderia, both of which contain a high concentration of hopanoid lipids, show high resistance to high temperature and low pH condition. Thus, in consideration of global warming and increasing soil acidity, both of those bacteria might outcompete other bacteria in soil and plant. The mutation strain shc, which produces no hopanoid lipids shows an inability in long-term colonization of a plant. Previous studies have found that in the beginning, this bacteria grow and develop in host normally. However, after a few days, this bacteria starts to degenerate and be recycled by their host.
Bacteria conduct the majority of nitrogen fixation in the low-oxygen concentration environment. Nitrogenase, the core enzyme in nitrogen fixation, is sensitive and vulnerable in the presence of oxygen. Thus, the majority of the nitrogen-fixing bacteria exist under the anaerobic environment. Sometimes, nitrogen fixation can also be conducted at high oxygen concentration. Under this condition, bacteria develop more sophisticated mechanisms to protect the nitrogenase and achieve nitrogen fixation. The free-living nitrogen-fixing bacteria, Azotobacter vinelandii, develop a various way to fix nitrogen under high oxygen condition. Previous studies observed that the activity of nitrogenase activity has negligible decrease under the air saturation from 30-100%. The Azotobacter vinelandii decrease their cellular surface area. Therefore, for each unit of cytoplasm, there is larger protection from the bacterial membrane. Also, under high oxygen condition, they also increase their oxygen consumption via a high respiration rate, thus creating an optional anaerobic environment for nitrogen fixation. The A. vinelandii also synthesize alginate to create alginate capsule, a thick barrier which limits the oxygen fission and protect the nitrogenase from the high oxygen concentrated environment. Under the lower oxygen concentrated environment, A. vinelandii develops a loose alginate capsule, while under the high concentration, they build up a compact and thick alginate capsule. Additionally, when the bacteria interacts with or inhabit plants, the host plants synthesize leghemoglobin molecules, one type of oxygen-transport metalloproteins. This hemoglobin scavenges oxygen from the root nodules of the leguminous plants, creating an optimal environment for nitrogenase activity (8).
Conclusion
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
