Kyasanur Forest Disease Virus: Difference between revisions
No edit summary |
|||
| (38 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Talbot2015}} | ||
=Classification= | =Classification= | ||
| Line 12: | Line 12: | ||
=Description and significance= | =Description and significance= | ||
Kyasanur forest disease virus (KFDV) is a tick-borne viral hemorrhagic fever causing virus | Kyasanur forest disease virus (KFDV) is a tick-borne viral hemorrhagic fever causing virus [[#References |[1]]]. which was first discovered in 1956, in the forests of southern India where it is endemic. Originally indigenous to the Shimoga district in Karnataka state, it has gradually emerged in other parts of southern Asia causing epidemics in recent decades [[#References |[2]]]. A major factor in its initial emergence was deforestation which brought monkeys into close contact with humans [[#References |[3]]]. KFDV was first isolated in sick monkeys inhabiting the Kyasanur forest during a series of epizootic outbreaks. Although many animal species appear to be resistant to infection by this virus, the black-faced langur (''Presbytis entellus''), the red-faced bonnet monkey (''Macaca radiata'') and humans are highly susceptible to infection by KFDV. ''Haemaphysalis spinigera'' ticks act as vectors for the virus. These ticks have a life cycle lasting approximately 12 months and involving three feeding stages. At each stage of this cycle they take a bloodmeal from mammals which if infected will transfer the virus to the feeding tick.When replete, ticks drop off the host animal landing in the moist undergrowth where they remain for several months undergoing transstadial changes to the next stage of their life cycle (from larvae, to nymphs and then to adults). When these infected ticks take their next bloodmeal, they may infect the host on which they feed. Humans with occupational exposure to forest animals (i.e., herders, farmers, forest workers, hunters) are especially at high risk of infection when they come into contact with the ticks on the animals or in the undergrowth. Consequently, KFD has become a concern for public health authorities within areas endemic for KFDV [[#References |[4]]]. | ||
Current studies of KFDV are focused on improvement of disease control strategies and molecular epidemiological analyses to track the dispersal of KFDV and close relatives outside of India. Methods for disease control include, i) understanding the modes of virus transmission, ii) developing effective rapid diagnostic tests, iii) improving the safety and effectiveness of available vaccines with which to protect humans from infection against KFD, and antiviral drug discovery to provide post-infection treatment (1, 2, 4, 5). Moreover, improved molecular methods for virus identification and characterisation have now demonstrated the presence of KFDV and the closely related Alkhurma hemorrhagic fever virus (AHFV) in Saudi Arabia | Current studies of KFDV are focused on improvement of disease control strategies and molecular epidemiological analyses to track the dispersal of KFDV and close relatives outside of India. Methods for disease control include, i) understanding the modes of virus transmission, ii) developing effective rapid diagnostic tests, iii) improving the safety and effectiveness of available vaccines with which to protect humans from infection against KFD, and antiviral drug discovery to provide post-infection treatment ([[#References |[1]]], [[#References |[2]]], [[#References |[4]]], [[#References |[5]]]). Moreover, improved molecular methods for virus identification and characterisation have now demonstrated the presence of KFDV and the closely related Alkhurma hemorrhagic fever virus (AHFV) in Saudi Arabia [[#References |[4]]]., China [[#References |[6]]] and Egypt [[#References |[7]]], suggesting that KFDV has its evolutionary origins in Africa [[#References |[7]]]. | ||
Despite current efforts to control and trace the movement of KFDV, particularly in India where the virus was originally believed to have its evolutionary origins, it is clear that this virus and its close relatives are causing increasing numbers of cases of hemorrhagic fever and in some cases encephalitis | Despite current efforts to control and trace the movement of KFDV, particularly in India where the virus was originally believed to have its evolutionary origins, it is clear that this virus and its close relatives are causing increasing numbers of cases of hemorrhagic fever and in some cases encephalitis [[#References |[8]]]. Indeed, with increasing global temperatures, increasing transportation of animals, humans and commercial goods across the world, the need to develop strategies to control the spread of these deadly pathogens and many related viruses is a growing challenge [[#References |[3]]]. | ||
=Genome structure= | =Genome structure= | ||
As part of the tick-borne encephalitis virus (TBEV) family Flaviviridae, this virus contains a spherical positive single-stranded RNA molecule of 11,000 bases in length (8 | As part of the tick-borne encephalitis virus (TBEV) family Flaviviridae, this virus contains a spherical positive single-stranded RNA molecule of 11,000 bases in length ([[#References |[8]]] [[#References |[9]]], [[#References |[10]]]). | ||
=Morphology= | =Morphology= | ||
The species Kyanasur Forest Disease virus is pathogenic member of the Flaviviridae family (genus flavivirus) and belongs to the tick-borne encephalitis virus group (TBEV). This group consists of related viruses such as the Louping ill, Omsk hemorrhagic fever, Langat, Royal Farm, Gadgets Gully, and Powassan viruses [[#References |[4]]]. There is a wide range of tick species contributing to the tick-borne KFDV group, mainly the Haemaphysalis spinigera. Mature TBEVs have a diameter of 50 nm and contain 3 structural proteins for the capsid, envelope and membrane [[#References |[9]]]. These viruses enter the cell via receptor-mediated endocytosis and undergo an immature and mature phase inside the cell [[#References |[9]]]. A recent study showed that infection of KFDV is lethal in mice and leads to elevated levels of pro-inflammatory cytokines in the brain. However, not virus was detected in visceral organs [[#References |[11]]]. | |||
=Ecology= | =Ecology= | ||
Other than Haemaphysalis spinigera, KFDV has been isolated from 16 different types of ticks. Wild monkeys, Presbytis entellus (Black faced langur) and Macaca radiata (Red faced bonnet monkey), are common in the forest localities that commonly get infected by KFDV through bites of infected ticks, acting as sentinel animals as they are susceptible to KFDV | Other than ''Haemaphysalis spinigera'', KFDV has been isolated from 16 different types of ticks. Wild monkeys, ''Presbytis entellus'' (Black faced langur) and ''Macaca radiata'' (Red faced bonnet monkey), are common in the forest localities that commonly get infected by KFDV through bites of infected ticks, acting as sentinel animals as they are susceptible to KFDV [[#References |[1]]]. KFDV also circulates in small animals such as rodents, shrews, and birds [[#References |[12]]]. The ecology of the Shimoga area is characterized by high humidity and is special due to the presence of divergent biotypes of forests, cultivated areas, and grasslands. The high humidity generated from the cultivated fields is favorable for tick maintenance throughout the year and availability of nearby forest sustains a large population of wild monkeys. Because such ecological specificity is rarely observed in other parts of India, this combination of ecological factors may be responsible for the geographical localization of KFDV only to Karnataka state of India [[#References |[13]]]. Tick-borne viral hemorrhagic fever, however, is also present throughout other parts of Eurasia as the TBEV viral group undergoes recombination events, creating different viruses better adapted to and that are easily spread [[#References |[3]]]. | ||
=Transmission= | =Transmission= | ||
Transmission of this virulent KFDV cycles through ticks, mammals, and birds; ticks are especially significant in maintaining the virus’ natural cycle ( | Transmission of this virulent KFDV cycles through ticks (figure 1), mammals, and birds; ticks are especially significant in maintaining the virus’ natural cycle ([[#References |[1]]], [[#References |[14]]]. It is most commonly seen in monkeys, when they are exposed to infectious tick bites. The ticks preserve the virus and remain infected for the duration of their lives, allowing them to pass on the virus to offspring as well. The vector ticks of KFDV remain on their host until their host experiences death. At that point, the ticks detach and create a hotspot for the virus to increase susceptibility to the subjects around the area and continue spreading [[#References |[2]]]. The virus has been noted to spread across different populations and regions, especially through birds migrating after infection. Animals are not the only victims of this virus as humans can also get infected, particularly those that are occasionally in forested areas and rural areas. Humans are at risk usually when they come into contact with infectious ticks or other infected animals [[#References |[1]]]. In most human cases, workers have suffered from the disease due to improper handling (i.e lack of or reckless use of biosafety gear) of infected monkeys in forested areas [[#References |[2]]]. Fortunately, researchers have not found any cases showing human-to-human transmittance as indicative of the spread of the disease [[#References |[1]]]. Humans are most likely dead end hosts as the KFD virus has not been found in any blood secretions, ruling out the probability of human-to-human transmittance [[#References |[4]]]. | ||
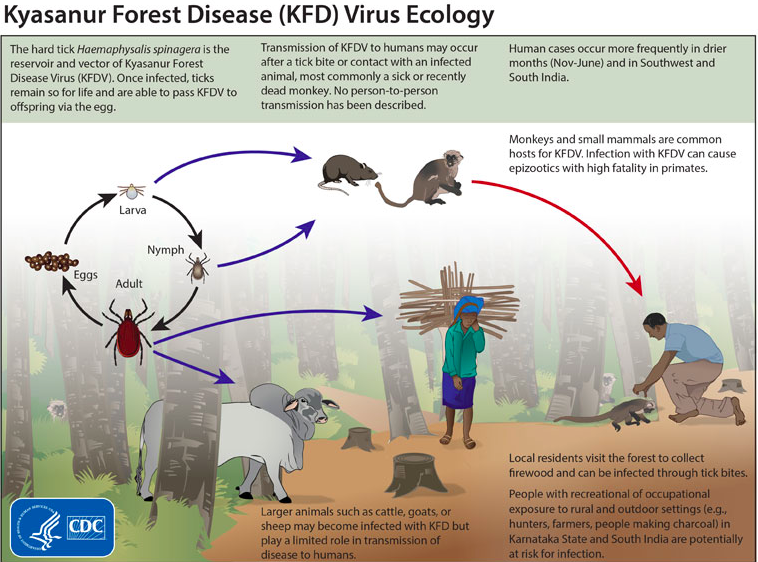
[[File:Kyasanur_forest_disease_virus.png|options|This figure was obtained from the CDC’s informational website on the Kyasanur forest disease virus.]] | |||
=Pathology and Diagnostics= | =Pathology and Diagnostics= | ||
KFDV has an incubation period of 3-8 days, and a rapid onset, which generally starts with chills, hemorrhagic fever, headaches, and physical weakness. During days 3-4, patients may experience myalgia (muscular pain), vomiting, and other gastrointestinal symptoms. Some cases can develop hemorrhages into the skin, viscera, and mucosa, abnormally low blood pressure, and low platelets level. Previous case-control studies have found that other common clinical manifestations of patients include bleeding, conjunctival congestion, hematuria, epistaxis, and hematemesis | KFDV has an incubation period of 3-8 days, and a rapid onset, which generally starts with chills, hemorrhagic fever, headaches, and physical weakness. During days 3-4, patients may experience myalgia (muscular pain), vomiting, and other gastrointestinal symptoms. Some cases can develop hemorrhages into the skin, viscera, and mucosa, abnormally low blood pressure, and low platelets level. Previous case-control studies have found that other common clinical manifestations of patients include bleeding, conjunctival congestion, hematuria, epistaxis, and hematemesis [[#References |[15]]]. Recovery usually happens without further complications after 1-2 weeks after symptoms appear. Nevertheless, a fraction of the patients (10-20%) experience a second phase of the disease, which consists mainly of neurological abnormalities, such as mental disturbances, tremors and vision difficulties. | ||
Since it was first identified, 400-500 outbreaks of KFDV occur per year spreading to adjacent regions of the Shimoga district. These outbreaks are seasonal, occurring mostly from January to June | Since it was first identified, 400-500 outbreaks of KFDV occur per year spreading to adjacent regions of the Shimoga district. These outbreaks are seasonal, occurring mostly from January to June [[#References |[15][16]]]. The case fatality rate is about 5%. Diagnosing this virus can be achieved in early stages of the illness using PCR for molecular detection. Isolation from blood could also be done. To detect the presence of the virus, enzyme-linked immunosorbent assay (ELISA) can be performed [[#References |[2]]]. | ||
=Vaccination and Prevention= | =Vaccination and Prevention= | ||
Vaccination with formalin-inactivated tissue-culture vaccine has been the primary strategy for controlling KFD in the five endemic districts of Karnataka since 1990 | Vaccination with formalin-inactivated tissue-culture vaccine has been the primary strategy for controlling KFD in the five endemic districts of Karnataka since 1990 [[#References |[1]]]. The strategy involves mass vaccination in areas reporting KFD activity (defined as laboratory evidence of confirmed cases in monkeys, humans, or ticks) and in villages within a 5 km radius of such areas. Two vaccine doses are administered at least 1 month apart to persons 7–65 years of age. Vaccine-induced immunity is short-lived, so the first booster dose of vaccine is recommended within 6–9 months after primary vaccination; thereafter, annual booster doses are recommended for 5 years after the last confirmed case in the area ([[#References |[17]]]. Besides vaccination, other preventative measures include utilization of insect repellents and wearing protective clothing to avoid tick bites [[#References |[14]]]. | ||
People residing in the forest areas, as well as those working in the parks, sanctuaries, and reserve forests remain at risk of acquiring the disease. As the presence of the disease often becomes noticeable when enzootic infections occur and sentinel animals such as monkeys start dying, establishing an event-based surveillance system for monkey deaths in the national parks, wildlife sanctuaries, reserve forests of the Western Ghats, and neighboring villages may help detect the disease early to institute appropriate control measures | People residing in the forest areas, as well as those working in the parks, sanctuaries, and reserve forests remain at risk of acquiring the disease. As the presence of the disease often becomes noticeable when enzootic infections occur and sentinel animals such as monkeys start dying, establishing an event-based surveillance system for monkey deaths in the national parks, wildlife sanctuaries, reserve forests of the Western Ghats, and neighboring villages may help detect the disease early to institute appropriate control measures. Conducting sero-surveys in different districts of the region may be a helpful measure for mapping of the disease [[#References |[1]]]. | ||
=Current Research in Related Viruses= | =Current Research in Related Viruses= | ||
The emergence of new viruses can be attributed to recombination events and the emergence of hybrids. Efforts have been made in order to sort these viruses phylogenetically as these viruses exhibit a high mutation frequency, quickly evolving | The emergence of new viruses can be attributed to recombination events and the emergence of hybrids. Efforts have been made in order to sort these viruses phylogenetically as these viruses exhibit a high mutation frequency, quickly evolving [[#References |[18]]]. Serological evidence of viruses related to KFDV has been found from isolates in western India, Saudi Arabia, and Africa [[#References |[3][7]]]. The origins, related flaviviruses, (as of 2008), including association of the viruses with their vectors, hosts and geographic distribution in either the Old World or New World are listed in Figure 2 [[File:Phylogenetic_tree_of_related_viruses.png|options|This Figure illustrates the phylogenetic relationships of KFDV with other related flaviviruses, (as of 2008), including association of the viruses with their vectors, hosts and geographic distribution in either the Old World or New World. Permission to use this image was obtained from Professor E A Gould, author of Pathogenic flaviviruses originally published in Lancet 2008; 371: 500-09]]. | ||
The Alkhurma Hemorrhagic Fever Virus (AHFV) is a zoonotic virus that affects mainly populations in the Arabian Peninsula. AHFV has high genetic similarity with KFDV, having an 89% sequence homology, suggesting a common ancestral origin | The Alkhurma Hemorrhagic Fever Virus (AHFV) is a zoonotic virus that affects mainly populations in the Arabian Peninsula. AHFV has high genetic similarity with KFDV, having an 89% sequence homology, suggesting a common ancestral origin [[#References |[19]]]. AHFV was initially isolated in 1995 from a patient in Saudi Arabia who died from this virus after having slaughtered a sheep in the city of Alkhurma. Since the first description of the virus was made, several hundreds of cases of AHFV have been reported, even in adjacent countries, such as Egypt. Transmission of this virus is not well understood yet. However, it is believed that people can become infected through a tick bite, or by contact with domestic animals or livestock. Similar to KFDV, human-to-human transmission has not yet been reported for AHFV [[#References |[19]]]. | ||
Not surprisingly, AHFV and KFDV have similar symptoms, although the symptoms information for AHFV is limited. Incubation period is thought to be as short as 2-4 days, with an initial presentation of flu-like symptoms followed by a second phase of neurologic and hemorrhagic symptoms. Common observations of hospitalized patients with AHFV include thrombocytopenia and leukopenia. Compared to KFD virus, AHF virus has symptoms that seem to be more associated with neurologic diseases. It also showed to have a lower mortality rate in laboratory mice, compared to the KFD virus [[#References |[19]]]. | |||
A similar virus was isolated in China in 1989. The Nanjianyin virus was first discovered in the Yunnan province, located in the Hengduan Mountain region of China, from the serum of a 38-year-old woman. According to previous serosurveys done from 1987 to 1990, this region had previously been exposed to KFDV. In fact, healthy residents of this region, as well as resident birds, rodents and monkeys, were found to have antibodies against the KFD virus. Using primers that are specific to the Flavivirus genus viruses, the Nanjianyin virus was found to be 99% homologous to KFDV [[#References |[6]]]. A comparison was also made between the Nanjianyin virus and the AFH virus, resulting in a 90.4% similarity between the two. These results showed that the Nanjianyin virus belongs to the same virus clade as the KFD virus [[#References |[6]]]. | |||
[[ | Recently, AHFV isolation in Egypt has supported previous evidence that the tick-borne encephalitic flavivirus serocomplex may have originated in Africa [[#References |[7]]]. Little is known about the virulence and clinical implications AHFV may have on the general population, yet another reason this research is significant [[#References |[20]]]. | ||
=References= | =References= | ||
1. Murhekar, M.V., et al., On the transmission pattern of Kyasanur Forest disease (KFD) in India. ''Infect Dis Poverty'', 2015. 4: 37. | [1] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4545326/ Murhekar, M.V., et al., On the transmission pattern of Kyasanur Forest disease (KFD) in India. ''Infect Dis Poverty'', 2015. 4: 37.] | ||
[2] [http://www.researchgate.net/publication/230637923_Diagnosis_of_Kyasanur_forest_disease_by_nested_RT-PCR_real-time_RT-PCR_and_IgM_capture_ELISA Mourya, D.T., et al., Diagnosis of Kyasanur forest disease by nested RT-PCR, real-time RT-PCR and IgM capture ELISA. ''J Virol Methods'', 2012. 186(1-2): p. 49-54.] | |||
[3] [http://www.sciencedirect.com/science/article/pii/S014067360860238X Gould EA, Solomon T. Pathogenic flaviviruses. ''Lancet'' 2008; 371: 500–509.] | |||
[4] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2819879/ Mehla, R., et al., Recent Ancestry Of Kyasanur Forest Disease Virus. ''Emerging Infectious Diseases'', 2009. 15(9): p. 1431-1437.] | |||
[5] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4545326/ Kasabi, Gudadappa S., et al., Coverage and Effectiveness of Kyasanur Forest Disease (KFD) Vaccine in Karnataka, South India, 2005–10. ''PLoS Negl Trop Dis PLoS Neglected Tropical Diseases'' 7.1 (2013): n. pag. Web.] | |||
[6] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2657630/ Wang J, Zhang H, Fu S, Wang H, Ni D, Nasci R, et al. Isolation of Kyasanur forest disease virus from febrile patient, Yunnan, China. ''Emerg Infect Dis.'' 2009. 15(2): 326–8. doi:10.3201/eid1502.080979.] | |||
[7] [http://wwwnc.cdc.gov/eid/article/17/8/pdfs/10-1858.pdf Charrel, Remi, and Ernest A. Gould. Alkhurma Hemorrhagic Fever in Travelers Returning from Egypt, 2010. ''Emerg. Infect. Dis. Emerging Infectious Diseases'' (2011): n. pag. Web.] | |||
[8] [http://onlinelibrary.wiley.com/doi/10.1002/rmv.495/abstract Pattnaik, P. (2006). Kyasanur forest disease: An epidemiological view in India. ''Reviews in Medical Virology'', 2006. 16 (3), 151-165.] | |||
[9] [http://www.researchgate.net/publication/225289700_Flavivirus_and_flavivirus_vaccines Heinz, Franz X., and Karin Stiasny. Flaviviruses and Flavivirus Vaccines. ''Vaccine'' 30.29 (2012): 4301-306. Web.] | |||
[10] [http://www.ncbi.nlm.nih.gov/pubmed/2174669 Chambers TJ, Hahn CS, Galler R. Rice CM. Flavivirus genome organization, expression, and replication. ''Annu. Rev. Microbiol.'' 1990;44:649–688.] | |||
10. | [11] [http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0002934 Sawatsky, Bevan, Alexander J. Mcauley, Michael R. Holbrook, and Dennis A. Bente. Comparative Pathogenesis of Alkhumra Hemorrhagic Fever and Kyasanur Forest Disease Viruses in a Mouse Model. ''PLoS Negl Trop Dis PLoS Neglected Tropical Diseases.'' 2014 Jun; 8(6): e2934] | ||
[12] Banerjee K. Kyasanur Forest disease. The Arbo-Viruses: Ecology and Epidemiology (Vol. 3), Monath TP (ed.). CRC Press: Boca Roton, FL, 1988; 93–116. | |||
[13] [http://www.researchgate.net/publication/17372773_Kyasanur_Forest_disease_ecologic_considerations Boshel-M J. Kyasanur forest disease: ecological considerations. ''Am J Trop Med Hyg'' 1969; 18: 67–80.] | |||
[14] [http://www.cdc.gov/vhf/kyasanur/ "Kyasanur Forest Disease (KFD)." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 23 Dec. 2013. Web. 20 Oct. 2015.] | |||
[15] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3559039/ Kasabi, Gudadappa S., Manoj V. Murhekar, Pragya D. Yadav, R. Raghunandan, S.K. Kiran, V.K. Sandhya, G.H. Channabasappa, Akhilesh C. Mishra, Devendra Tarachand Mourya, and Sanjay M. Mehendale. "Kyasanur Forest Disease, India, 2011–2012." ''Emerging Infectious Diseases''. Centers for Disease Control and Prevention, Feb. 2013. Web. 04 Dec. 2015.] | |||
[16] [http://onlinelibrary.wiley.com/doi/10.1525/maq.1987.1.4.02a00040/abstract Nichter, Mark Kyasanur Forest Disease: An Ethnography of a Disease of Development. ''Medical Anthropology Quarterly.'' New Series, Vol. 1, No. 4 (Dec., 1987), pp. 406-423.] | |||
[17] [http://wwwnc.cdc.gov/eid/article/21/1/14-1227_article Kiran, S.K., et al., Kyasanur Forest disease outbreak and vaccination strategy, Shimoga District, India, 2013-2014. ''Emerg Infect Dis'', 2015. 21(1): p. 146-9.] | |||
[18] [http://link.springer.com/chapter/10.1007/978-1-4615-5331-1_93#page-1 Domingo, E., Escarmis, C., Sevilla, N. & Martinez, M.-A. (1997) Population dynamics and molecular evolution of RNA viruses. ''Factors in the Emergence of Arbovirus Diseases'', pp. 273–278. Edited by J. F. Saluzzo & B. Dodet. Paris: Elsevier.] | |||
[19] [http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0001352 Dodd, Kimberly, Brian Bird, Marina Khirstova, Cesar Albariño, Serena Carroll, James Corner, Bobbie Erickson, Pierre Rollin, and Stuart Nichol. Ancient Ancestry of KFDV and AHFV Revealed by Complete Genome Analyses of Viruses Isolated from Ticks and Mammalian Hosts. ''PLOS Neglected Tropical Diseases: PLOS.'' (2011); 10.1371] | |||
[20] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3381587/ Carletti F, Castilletti C, Di Caro A, Capobianchi MR, Nisii C, Suter F, et al. Alkhurma hemorrhagic fever in travelers returning from Egypt, 2010. ''Emerg Infect Dis.'' 2010;16:1979–82.] | |||
<br><br> | |||
<br>Edited by [Selene Ayuso, Kerlly Castellano, Jane Le, Anapatricia Maldonado], student of [mailto:jmtalbot@bu.edu Jennifer Talbot] for [http://www.bu.edu/academics/cas/courses/cas-bi-311/ BI 311 General Microbiology], 2015, [http://www.bu.edu/ Boston University]. | |||
Latest revision as of 15:25, 12 February 2016
Classification
Higher Order Taxa
- Group: IV
- Order: unassigned
- Family: Flaviviridae
- Genus: flavivirus
Species
- Kyasanur forest disease virus
Description and significance
Kyasanur forest disease virus (KFDV) is a tick-borne viral hemorrhagic fever causing virus [1]. which was first discovered in 1956, in the forests of southern India where it is endemic. Originally indigenous to the Shimoga district in Karnataka state, it has gradually emerged in other parts of southern Asia causing epidemics in recent decades [2]. A major factor in its initial emergence was deforestation which brought monkeys into close contact with humans [3]. KFDV was first isolated in sick monkeys inhabiting the Kyasanur forest during a series of epizootic outbreaks. Although many animal species appear to be resistant to infection by this virus, the black-faced langur (Presbytis entellus), the red-faced bonnet monkey (Macaca radiata) and humans are highly susceptible to infection by KFDV. Haemaphysalis spinigera ticks act as vectors for the virus. These ticks have a life cycle lasting approximately 12 months and involving three feeding stages. At each stage of this cycle they take a bloodmeal from mammals which if infected will transfer the virus to the feeding tick.When replete, ticks drop off the host animal landing in the moist undergrowth where they remain for several months undergoing transstadial changes to the next stage of their life cycle (from larvae, to nymphs and then to adults). When these infected ticks take their next bloodmeal, they may infect the host on which they feed. Humans with occupational exposure to forest animals (i.e., herders, farmers, forest workers, hunters) are especially at high risk of infection when they come into contact with the ticks on the animals or in the undergrowth. Consequently, KFD has become a concern for public health authorities within areas endemic for KFDV [4].
Current studies of KFDV are focused on improvement of disease control strategies and molecular epidemiological analyses to track the dispersal of KFDV and close relatives outside of India. Methods for disease control include, i) understanding the modes of virus transmission, ii) developing effective rapid diagnostic tests, iii) improving the safety and effectiveness of available vaccines with which to protect humans from infection against KFD, and antiviral drug discovery to provide post-infection treatment ([1], [2], [4], [5]). Moreover, improved molecular methods for virus identification and characterisation have now demonstrated the presence of KFDV and the closely related Alkhurma hemorrhagic fever virus (AHFV) in Saudi Arabia [4]., China [6] and Egypt [7], suggesting that KFDV has its evolutionary origins in Africa [7].
Despite current efforts to control and trace the movement of KFDV, particularly in India where the virus was originally believed to have its evolutionary origins, it is clear that this virus and its close relatives are causing increasing numbers of cases of hemorrhagic fever and in some cases encephalitis [8]. Indeed, with increasing global temperatures, increasing transportation of animals, humans and commercial goods across the world, the need to develop strategies to control the spread of these deadly pathogens and many related viruses is a growing challenge [3].
Genome structure
As part of the tick-borne encephalitis virus (TBEV) family Flaviviridae, this virus contains a spherical positive single-stranded RNA molecule of 11,000 bases in length ([8] [9], [10]).
Morphology
The species Kyanasur Forest Disease virus is pathogenic member of the Flaviviridae family (genus flavivirus) and belongs to the tick-borne encephalitis virus group (TBEV). This group consists of related viruses such as the Louping ill, Omsk hemorrhagic fever, Langat, Royal Farm, Gadgets Gully, and Powassan viruses [4]. There is a wide range of tick species contributing to the tick-borne KFDV group, mainly the Haemaphysalis spinigera. Mature TBEVs have a diameter of 50 nm and contain 3 structural proteins for the capsid, envelope and membrane [9]. These viruses enter the cell via receptor-mediated endocytosis and undergo an immature and mature phase inside the cell [9]. A recent study showed that infection of KFDV is lethal in mice and leads to elevated levels of pro-inflammatory cytokines in the brain. However, not virus was detected in visceral organs [11].
Ecology
Other than Haemaphysalis spinigera, KFDV has been isolated from 16 different types of ticks. Wild monkeys, Presbytis entellus (Black faced langur) and Macaca radiata (Red faced bonnet monkey), are common in the forest localities that commonly get infected by KFDV through bites of infected ticks, acting as sentinel animals as they are susceptible to KFDV [1]. KFDV also circulates in small animals such as rodents, shrews, and birds [12]. The ecology of the Shimoga area is characterized by high humidity and is special due to the presence of divergent biotypes of forests, cultivated areas, and grasslands. The high humidity generated from the cultivated fields is favorable for tick maintenance throughout the year and availability of nearby forest sustains a large population of wild monkeys. Because such ecological specificity is rarely observed in other parts of India, this combination of ecological factors may be responsible for the geographical localization of KFDV only to Karnataka state of India [13]. Tick-borne viral hemorrhagic fever, however, is also present throughout other parts of Eurasia as the TBEV viral group undergoes recombination events, creating different viruses better adapted to and that are easily spread [3].
Transmission
Transmission of this virulent KFDV cycles through ticks (figure 1), mammals, and birds; ticks are especially significant in maintaining the virus’ natural cycle ([1], [14]. It is most commonly seen in monkeys, when they are exposed to infectious tick bites. The ticks preserve the virus and remain infected for the duration of their lives, allowing them to pass on the virus to offspring as well. The vector ticks of KFDV remain on their host until their host experiences death. At that point, the ticks detach and create a hotspot for the virus to increase susceptibility to the subjects around the area and continue spreading [2]. The virus has been noted to spread across different populations and regions, especially through birds migrating after infection. Animals are not the only victims of this virus as humans can also get infected, particularly those that are occasionally in forested areas and rural areas. Humans are at risk usually when they come into contact with infectious ticks or other infected animals [1]. In most human cases, workers have suffered from the disease due to improper handling (i.e lack of or reckless use of biosafety gear) of infected monkeys in forested areas [2]. Fortunately, researchers have not found any cases showing human-to-human transmittance as indicative of the spread of the disease [1]. Humans are most likely dead end hosts as the KFD virus has not been found in any blood secretions, ruling out the probability of human-to-human transmittance [4].
Pathology and Diagnostics
KFDV has an incubation period of 3-8 days, and a rapid onset, which generally starts with chills, hemorrhagic fever, headaches, and physical weakness. During days 3-4, patients may experience myalgia (muscular pain), vomiting, and other gastrointestinal symptoms. Some cases can develop hemorrhages into the skin, viscera, and mucosa, abnormally low blood pressure, and low platelets level. Previous case-control studies have found that other common clinical manifestations of patients include bleeding, conjunctival congestion, hematuria, epistaxis, and hematemesis [15]. Recovery usually happens without further complications after 1-2 weeks after symptoms appear. Nevertheless, a fraction of the patients (10-20%) experience a second phase of the disease, which consists mainly of neurological abnormalities, such as mental disturbances, tremors and vision difficulties.
Since it was first identified, 400-500 outbreaks of KFDV occur per year spreading to adjacent regions of the Shimoga district. These outbreaks are seasonal, occurring mostly from January to June [15][16]. The case fatality rate is about 5%. Diagnosing this virus can be achieved in early stages of the illness using PCR for molecular detection. Isolation from blood could also be done. To detect the presence of the virus, enzyme-linked immunosorbent assay (ELISA) can be performed [2].
Vaccination and Prevention
Vaccination with formalin-inactivated tissue-culture vaccine has been the primary strategy for controlling KFD in the five endemic districts of Karnataka since 1990 [1]. The strategy involves mass vaccination in areas reporting KFD activity (defined as laboratory evidence of confirmed cases in monkeys, humans, or ticks) and in villages within a 5 km radius of such areas. Two vaccine doses are administered at least 1 month apart to persons 7–65 years of age. Vaccine-induced immunity is short-lived, so the first booster dose of vaccine is recommended within 6–9 months after primary vaccination; thereafter, annual booster doses are recommended for 5 years after the last confirmed case in the area ([17]. Besides vaccination, other preventative measures include utilization of insect repellents and wearing protective clothing to avoid tick bites [14].
People residing in the forest areas, as well as those working in the parks, sanctuaries, and reserve forests remain at risk of acquiring the disease. As the presence of the disease often becomes noticeable when enzootic infections occur and sentinel animals such as monkeys start dying, establishing an event-based surveillance system for monkey deaths in the national parks, wildlife sanctuaries, reserve forests of the Western Ghats, and neighboring villages may help detect the disease early to institute appropriate control measures. Conducting sero-surveys in different districts of the region may be a helpful measure for mapping of the disease [1].
Current Research in Related Viruses
The emergence of new viruses can be attributed to recombination events and the emergence of hybrids. Efforts have been made in order to sort these viruses phylogenetically as these viruses exhibit a high mutation frequency, quickly evolving [18]. Serological evidence of viruses related to KFDV has been found from isolates in western India, Saudi Arabia, and Africa [3][7]. The origins, related flaviviruses, (as of 2008), including association of the viruses with their vectors, hosts and geographic distribution in either the Old World or New World are listed in Figure 2 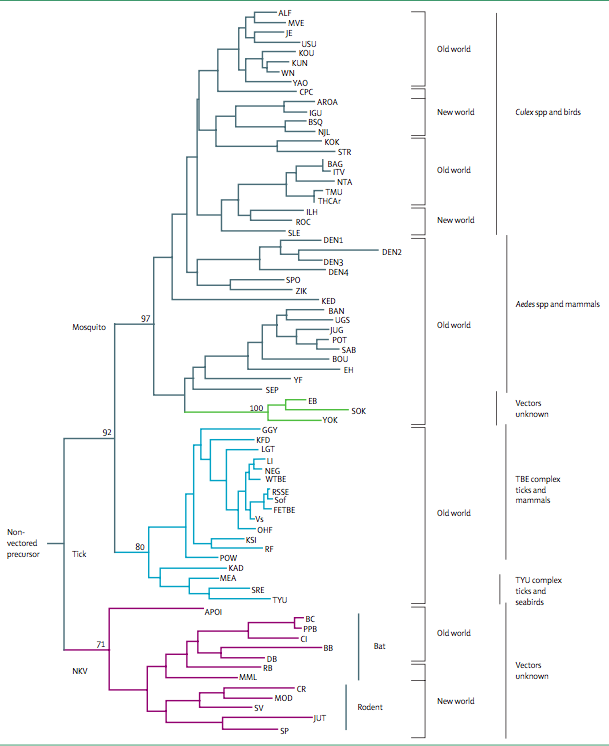 .
.
The Alkhurma Hemorrhagic Fever Virus (AHFV) is a zoonotic virus that affects mainly populations in the Arabian Peninsula. AHFV has high genetic similarity with KFDV, having an 89% sequence homology, suggesting a common ancestral origin [19]. AHFV was initially isolated in 1995 from a patient in Saudi Arabia who died from this virus after having slaughtered a sheep in the city of Alkhurma. Since the first description of the virus was made, several hundreds of cases of AHFV have been reported, even in adjacent countries, such as Egypt. Transmission of this virus is not well understood yet. However, it is believed that people can become infected through a tick bite, or by contact with domestic animals or livestock. Similar to KFDV, human-to-human transmission has not yet been reported for AHFV [19].
Not surprisingly, AHFV and KFDV have similar symptoms, although the symptoms information for AHFV is limited. Incubation period is thought to be as short as 2-4 days, with an initial presentation of flu-like symptoms followed by a second phase of neurologic and hemorrhagic symptoms. Common observations of hospitalized patients with AHFV include thrombocytopenia and leukopenia. Compared to KFD virus, AHF virus has symptoms that seem to be more associated with neurologic diseases. It also showed to have a lower mortality rate in laboratory mice, compared to the KFD virus [19].
A similar virus was isolated in China in 1989. The Nanjianyin virus was first discovered in the Yunnan province, located in the Hengduan Mountain region of China, from the serum of a 38-year-old woman. According to previous serosurveys done from 1987 to 1990, this region had previously been exposed to KFDV. In fact, healthy residents of this region, as well as resident birds, rodents and monkeys, were found to have antibodies against the KFD virus. Using primers that are specific to the Flavivirus genus viruses, the Nanjianyin virus was found to be 99% homologous to KFDV [6]. A comparison was also made between the Nanjianyin virus and the AFH virus, resulting in a 90.4% similarity between the two. These results showed that the Nanjianyin virus belongs to the same virus clade as the KFD virus [6].
Recently, AHFV isolation in Egypt has supported previous evidence that the tick-borne encephalitic flavivirus serocomplex may have originated in Africa [7]. Little is known about the virulence and clinical implications AHFV may have on the general population, yet another reason this research is significant [20].
References
[3] Gould EA, Solomon T. Pathogenic flaviviruses. Lancet 2008; 371: 500–509.
[12] Banerjee K. Kyasanur Forest disease. The Arbo-Viruses: Ecology and Epidemiology (Vol. 3), Monath TP (ed.). CRC Press: Boca Roton, FL, 1988; 93–116.
[13] Boshel-M J. Kyasanur forest disease: ecological considerations. Am J Trop Med Hyg 1969; 18: 67–80.
Edited by [Selene Ayuso, Kerlly Castellano, Jane Le, Anapatricia Maldonado], student of Jennifer Talbot for BI 311 General Microbiology, 2015, Boston University.