Metallosphaera cuprina: Difference between revisions
Jenn.wibowo (talk | contribs) |
Jenn.wibowo (talk | contribs) |
||
| Line 32: | Line 32: | ||
When grown on potassium tetrathionate-supplemented “Allen” plates, <i>M. cuprina</i> appears as rounded, convex colonies between 0.2-0.3 mm with a semi-transparent and smooth appearance. When supplemented with FeSO<sub>4</sub> instead of potassium tetrathionate, the colonies are brown and flat. [1]<br> | When grown on potassium tetrathionate-supplemented “Allen” plates, <i>M. cuprina</i> appears as rounded, convex colonies between 0.2-0.3 mm with a semi-transparent and smooth appearance. When supplemented with FeSO<sub>4</sub> instead of potassium tetrathionate, the colonies are brown and flat. [1]<br> | ||
Its cellular lipids are mainly composed of calditoglycerocaldarchaeol and caldarchaeol—the same core lipids that are also present in <i>M. sedula</i>, <i>M. hakonensis</i>, and <i>S. acidocaldarius</i> [1]. Caldarchaeol provide stability to the cell membrane and are present in many hyperthermophilic archaea [11]. While calditoglycerocaldarchaeol is also present in many | Its cellular lipids are mainly composed of calditoglycerocaldarchaeol and caldarchaeol—the same core lipids that are also present in <i>M. sedula</i>, <i>M. hakonensis</i>, and <i>S. acidocaldarius</i> [1]. Caldarchaeol provide stability to the cell membrane and are present in many hyperthermophilic archaea [11]. While calditoglycerocaldarchaeol is also present in many Sulfolobales, it might not be required for survival in thermoacidophilic environments. [12]<br> | ||
<i>M. cuprina</i> is an aerobic and facultatively chemolithoautotrophic species also capable of organotrophic growth on various organic materials. <i>M. cuprina</i> is able to use sulfidic ore as a source of metal ions, in addition to oxidizing reduced sulfur compounds. [1] Therefore, along with <i>M. sedula</i>, <i>M. cuprina</i> has a good degree of physiological versatility [7]. <i>M. cuprina</i> is capable of chemolithoautotrophic growth using elemental sulfur, K<sub2</sub>S<sub>4</sub>O<sub>6</sub>, and FeSO<sub>4</sub>. Similar to most reported members of the <i>Metallosphaera</i> genus, <i>M. cuprina</i> is capable of using tetrationate and pyrite or FeS as sulfur sources; L-Aspartic acid, L-Glutamic acid, L-Tryptophan, and L-Alanine as amino acid sources; and yeast extract, beef extract, peptone, tryptone, and Casamino acids as organic substrates. Unlike its fellow genus members, <i>M. cuprina</i> is capable of using L-Arabinose, D-Xylose, D-gGlucose, sucrose, and raffinose as sugars. As in <i>M. sedula</i>, <i>M. cuprina</i> is incapable of using D-Mannose for sugars. [1]<br> | |||
M. cuprina has a complete TCA cycle and an incomplete pentose phosphate pathway. [2] | M. cuprina has a complete TCA cycle and an incomplete pentose phosphate pathway. [2] | ||
Revision as of 07:24, 15 December 2012
Classification
Domain: Archaea
Phylum: Crenarchaeota
Class: Thermoprotei
Order: Sulfolobales
Family: Sulfolobaceae
Genus: Metallosphaera
Description and Significance
Metallosphaera cuprina was originally isolated from muddy hot spring water in the Yunnan province of China and is named in reference to copper, cuprina, due to the extraction of copper from ores near the hotspring. [1]
M. cuprina differs from other species of Metallosphaera as it is more extermophilic and can grow in lower temperatures and higher pH than most. M. cuprina grows best at 65°C, and pH 3.5, but can grow in ranges of 0-1% (w/v) NaCl, 55-75° C, and pH 2.5-5.5. [1]
There is potential for the use of M. cuprina and other Metallosphaera in the mining industry through bioleaching, due to its oxidation of reduced inorganic sulfur compounds. [2] Bacteria are typically used to further oxidize Fe3+-oxidized ore, and regenerate Fe2+. [15]
Genome Structure and Phylogeny
The M. cuprina genome is 1.84Mb [2] and contains 2077 genes [9]. In comparison, Metallosphaera sedula has a 2.19 Mb genome and 2307 genes [16], making the M. cuprina genome16% smaller than M. sedula.
The Ar-4T strain of M. cuprina has a GC content of 40.2 mol%. This is slightly lower than other Metallosphaera species: M. sedula, 46.2 mol% [8]; and Metallosphaera hakonensis, 46.2 mol%[10]; Metallosphaera prunae, 46 mol%[4].
This strain shares sequence similarities of 97.7% with M. hakonensis DSM 7519T, 97.0% with M. sedula DSM 5348T, and 96.8% with M. prunae DSM 10039T. [1]
Through 16S rRNA gene sequence analysis, it is observed that M. cuprina shares less than 90% similarities with the Acidianus genera, and less than 88% similarities with the Sulfolobus genera. [1]
Cell Structure, Metabolism and Life Cycle
M. cuprina is a Gram-negative, irregular cocci of 0.9-1.0µm diameter [1]. Like M. prunae[4] but not M. hakonensis or M. sedula [1][5][10], M. cuprina has flagella and is motile [4]. The flagella of M. cuprina are long and curved. [1]
When grown on potassium tetrathionate-supplemented “Allen” plates, M. cuprina appears as rounded, convex colonies between 0.2-0.3 mm with a semi-transparent and smooth appearance. When supplemented with FeSO4 instead of potassium tetrathionate, the colonies are brown and flat. [1]
Its cellular lipids are mainly composed of calditoglycerocaldarchaeol and caldarchaeol—the same core lipids that are also present in M. sedula, M. hakonensis, and S. acidocaldarius [1]. Caldarchaeol provide stability to the cell membrane and are present in many hyperthermophilic archaea [11]. While calditoglycerocaldarchaeol is also present in many Sulfolobales, it might not be required for survival in thermoacidophilic environments. [12]
M. cuprina is an aerobic and facultatively chemolithoautotrophic species also capable of organotrophic growth on various organic materials. M. cuprina is able to use sulfidic ore as a source of metal ions, in addition to oxidizing reduced sulfur compounds. [1] Therefore, along with M. sedula, M. cuprina has a good degree of physiological versatility [7]. M. cuprina is capable of chemolithoautotrophic growth using elemental sulfur, K<sub2S4O6, and FeSO4. Similar to most reported members of the Metallosphaera genus, M. cuprina is capable of using tetrationate and pyrite or FeS as sulfur sources; L-Aspartic acid, L-Glutamic acid, L-Tryptophan, and L-Alanine as amino acid sources; and yeast extract, beef extract, peptone, tryptone, and Casamino acids as organic substrates. Unlike its fellow genus members, M. cuprina is capable of using L-Arabinose, D-Xylose, D-gGlucose, sucrose, and raffinose as sugars. As in M. sedula, M. cuprina is incapable of using D-Mannose for sugars. [1]
M. cuprina has a complete TCA cycle and an incomplete pentose phosphate pathway. [2]
Ecology and Pathogenesis
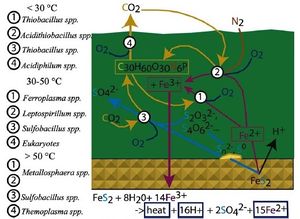
M. sedula can be found in sulfur rich hot springs, volcanic fields, and in acid mine drainage (AMD) communities. These communities are characterized by high metal ion concentrations, low pH and high temperatures. [1] [9] [10]
Though the dissolution of pyrite in AMD is a natural process, it is accelerated the presence of acidophiles such as M. sedula that are found in these environments, thus leading to increased rates of acidification of water draining for active and abandoned mines. AMD communities are characterized by a diverse composition of microorganisms that fill available niches depending on their tolerance to temperature, metal resistance and pH. These communities display a complex symbiosis through the biogeochemical cycling of sulfur, iron, carbon and nitrogen. At high temperatures, M. sedula fills the niche of iron and sulfur oxidizer, a role that is filled by other acidophiles such as the mesophilic Ferroplasma spp and Leptospirillum spp at lower temperatures. [10]
References
1. Huber, G. ,Spinnler, C. , Gambacorta , A., and Stetter, K. “Metallosphaera sedula gen. and sp. nov. Represents a New Genus of Aerobic, Metal-Mobilizing, Thermoacidophilic Archaebacteria”. Systematic and Applied Microbiology. 1989. p. 38-47.
2. Auernik, K., and Kelly, R. “Physiological Versatility of the Extremely Thermoacidophilic Archaeon Metallosphaera sedula Supported by Transcriptomic Analysis of Heterotrophic, Autotrophic, and Mixotrophic Growth”. Applied and Environmental Microbiology. 2010. p. 931-935.
3. Clark, T., Baldi, F., And Olson, G. “Coal Depyritization by the Thermophilic Archaeon Metallosphaera sedula”. Applied and Environmental Microbiology. 1993. p. 2375-2379.
4. http://www.epa.gov/oaqps001/sulfurdioxide/
5. http://www.epa.gov/acidrain/what/index.html
6. Peeples, T.L., and Kelly, R.M., “Bioenergetics of the metal/sulfur-oxidizingextreme thermoacidophile, Metallosphaera sedula”. Fuel. 1993. p. 1577-1752.
7. Auernik, K and Kelly, R. “Impact of Molecular Hydrogen on Chalcopyrite Bioleaching by the Extremely Thermoacidophilic Archaeon Metallosphaera sedula”. Applied and Environmental Microbiology. 2010. p. 2668-2672.
8. Alber, B., Kung, J., and Fuchs, G. "3-Hydroxypropionyl-Coenzyme A Synthetase from Metallosphaera sedula, an Enzyme Involved in Autotrophic CO2 Fixation". Journal of Bacteriology 2008. p. 1383-1389
9. http://genome.jgi-psf.org/metse/metse.home.html
10. Baker, B., and Banfield, J. "Microbial Communities in Acid Mine Drainage". FEMS Microbial Ecology. 2002. p. 139-152
11. Auernick, K. S., Maezato, Y., Blum, P. H., Kelly, R. M. “The Genome Sequence of the Metal-Mobilizing, Extremely Thermoacidophilic Archaeon Metallosphaera sedula Provides Insights into Bioleaching-Associated Metabolism”. Applied and Environmental Microbiology. 2008. p. 682-692
Author
Page authored by Stephanie Napieralski and Caitlin Miller, students of Prof. Jay Lennon at Michigan State University.
<-- Do not remove this line-->