Microbial biofilm inhibits wound healing
Introduction
By Katie Kaestner
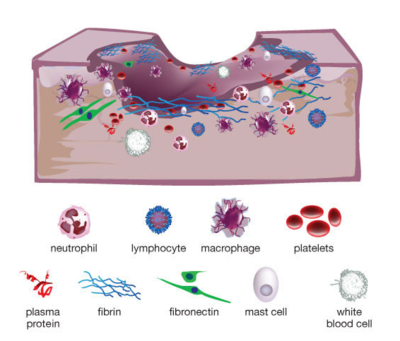
When someone suffers an injury to the skin, such as a laceration or an abrasion, the tissue normally undergoes four basic stages of wound healing. The inflammatory phase initiates the process, during which the body’s immune system sends nutrients, platelets, white blood cells (namely neutrophils, lymphocytes, and macrophages), antibodies, enzymes, and other immune factors to the affected area to establish homeostasis and prevent infection. Keratinocytes and inflammatory cytokines are then recruited to the area as the wound enters the epithelialization phase, providing temporary protection and activating the next phase of proliferation. A scab is a result of the epithelialization stage. After a few days, fibroblasts and collagen regenerate tissue and blood vessels in the proliferation stage, until finally reaching the remodeling stage in which collagen is continually formed and lysed until the skin is restored to its original strength.[1]
However, some wounds may be arrested in the inflammatory phase (Figure 1). These wounds, referred to as chronic wounds, may take weeks, months, or years to heal, and become highly susceptible to bacterial infection without persistent care. Chronic wound infections make up 60 - 80% of all human infectious diseases, and are cause of major concern to global health in view of our aging population and increasing prevalence of diabetes mellitus and obesity.[2] One in 20 elderly people live with chronic wounds resulting from diabetes or poor circulation, especially those confined to a wheelchair or a bed.[3] Chronic wounds in diabetic patients can be especially fatal. Every year, chronic wound infections in diabetics lead to over 70,000 lower-leg amputations in the United States alone, and up to half of these patients die within the first 18 months following the procedure. Of those who survive, half lose their contralateral extremity within 5 years.[4] As an estimated 415 million adults currently have diabetes and this number is projected to increase to 642 million by year 2040, developing treatment and prevention methods for chronic wound and infection is and will continue to be crucial to reduce medical costs and improve human health.[5]
As common as the health problem is, however, current chronic wound treatments are insufficient and too often lead to amputation due to a lack of understanding of the microbiology of these infections or how to eliminate them. Although it was known that microbial infections significantly complicate and delay wound healing processes, recent research suggests that the presence of microbial biofilms, as opposed to free-swimming planktonic bacteria, contribute significantly to the chronicity of a wound. Over 90% of chronic wounds are infected with biofilms, while biofilms are found in only 6% of acute wounds.[6] The protective and hostile nature of these biofilms renders their complete removal from the wound bed of chronic infections extremely difficult.
These findings lead to a series of intriguing questions. Why do biofilms develop in chronic wounds? Is it the environment of the chronic wound that promotes biofilm formation, or is it the development of a biofilm that causes a wound to become chronic? What are the current methods of treatment of biofilm-associated chronic wounds, and what are the characteristics of biofilms that so often render these treatments inadequate to heal the wound? What are other ways in which researchers and medical providers can explore chronic wound treatment?
This investigation of biofilm formation in chronic wounds will place a special focus on the opportunistic pathogen Pseudomonas aeruginosa and diabetes-associated chronic wounds, as these two areas in microbiology have been well-studied and present good models for relationships between biofilm formation and chronicity of wounds. P. aeruginosa is notorious for its tendency to form resilient biofilms and is the most commonly isolated pathogen from chronic wound infections.[7]
Biofilm General Structure and Properties
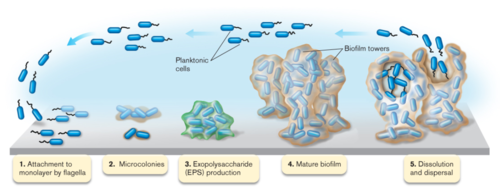
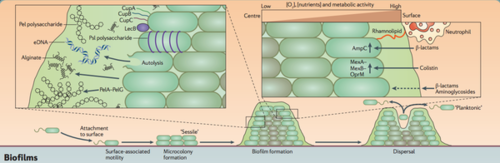
Most bacteria in nature aggregate to form three-dimensional, multicellular communities firmly adhered to a substrate or surface called biofilms. If free-swimming, planktonic bacteria discover a favorable environment abundant in nutrients, they will form sessile biofilms to settle in that favorable environment. A biofilm “life cycle” involves four general stages (Figure 2). The cells adhere to a substrate and form microcolonies, coating the substrate with polysaccharides or glycoproteins for more cells to attach. As more and more cells aggregate, the concentration of chemical signals reaches a point that triggers genetic changes in the cells that cause them to bind tightly to the surface and to neighboring cells. These microcolonies produce a thick extracellular matrix composed of exopolysaccharides (EPS) that forms a protective physical barrier around the bacteria, allowing them to grow into a mature biofilm of complex communities capable of chemical communication, a process called quorum sensing (QS). QS systems allow bacteria to, in effect, “sense” the presence of other bacteria by their secreted chemical signals. Once the biofilm reaches a particular cell density, a point of saturation, the biofilm turns off genes producing EPS products and reactivates flagellar motility genes to disperse new planktonic cells from the disseminating biofilm in search of a new environment.[8] P. aeruginosa, for example, produces EPSs such as alginate, Pel, and Psl (Figure 3). When the microcolonies of the biofilm begins to deplete its source of nutrients or oxygen, P. aeruginosa secretes an enzyme alginate lyase that dissolves the EPS in preparation for dispersal.
Microbial biofilms can be found in many environments, such as in the soil, plant roots, the human body, or on industrial materials in wet environments. And not all of these biofilms are pathogenic to the host or surrounding environment; in fact, most biofilm-forming bacteria involved in infection are species of normal nonpathogenic microflora, and can even play a protective and beneficial role in the host. Recent research has found that biofilm formation by vaginal lactobacilli provide protection against infectious bacteria.[9] But the balance in microbial composition of nonpathogenic biofilms are fragile; when disturbed, opportunistic pathogens can cause a biofilm to become dangerously noxious. Biofilms are particularly a concern when they develop on medical instruments or devices in the human body during treatments (Figure 4). A biofilm that contaminates an intravascular catheter can cause dispersal cells to be carried into the bloodstream of a patient as it delivers fluids and medications, causing a serious, life-threatening infection called septicemia, or sepsis.[10]

It is important to keep in mind that while some biofilms may be predominantly of a single species, most biofilms in nature are polymicrobial, composed of a plethora of different species. The greater special diversity of a biofilm, the better the polymicrobial communities can develop beneficial synergistic relationships to increase its chance of survival.
So why do biofilms frequently develop in chronic wounds and less so in acute wounds? Is it the environment of the chronic wound that stimulates biofilm formation, or is it rather the presence of the biofilm that causes the chronicity of a wound? Studies suggest that the answer is a bit of both - the chronic wound environment is highly hospitable for opportunistic pathogens to form biofilms, and that the properties inherent to biofilms subsequently perpetuates the inflammatory stage in wound healing, prolonging and severely advancing the chronicity of the infection.
Neuropathy is a condition of peripheral nerve damage that develops in 70% of diabetics, causing weakness and/or numbness in the extremities. A diabetic experiencing neuropathy will often suffer an injury to the skin tissue, and, unable to feel or see the injury (especially in the plantar region of the foot), the patient will fail to detect the wound before it becomes chronic.[11] The necrotic tissue and debris that develops in the wound bed before its detection provide the ideal substrate for bacteria to attach and form biofilms. As the wound goes undetected, the accumulation of bacteria further impedes the wound’s ability to heal, inducing the immune system to prolong the inflammatory stage. The interaction of the biofilm with the patient’s immune system perpetuates the chronicity of the wound in a vicious cycle, and the physical and chemical protective properties of the biofilm complicate its removal and prevent antibiotics from penetrating its surface. Moreover, improper or inadequate chronic wound treatment can quickly increase the biofilm’s resistance to them. The following sections will discuss the physical and chemical properties of the interaction between microbial biofilms and host immune system components in a chronic wound, and describe current detection and treatment methods and assess the advantages and limitations of each.
Biofilms Delay Wound Healing
Three main properties of biofilms contribute to the chronicity of wound infections. First, the biofilm’s durable and persistent presence leads to the continuous presentation of antigens that are recognized by the host immune system, overstimulating immune responses that, in turn, provide nutrients to the bacteria of the biofilm for further growth and damages nearby, healthy tissue. As long as the biofilm remains, the immune system continually tries to remove the biofilm and perpetuates the inflammatory phase. Second, the structure and composition of the biofilm extracellular EPS matrix acts as a mechanical barrier against penetration by immune cells and antibiotics, as well as facilitating rapid resistance to debridement (mechanical removal) and antibiotics. The matrix also inhibits the healing process by preventing key wound healing components such as fibroblasts and keratinocytes from entering the wound bed. And third, the diversity of species involved in pathogenic biofilms make it increasingly challenging for finding the appropriate and most effective treatment of chronic wounds, as species that are typically nonpathogenic and susceptible to antibiotics gain antibiotic resistance under the protection of the biofilm and neighboring resistant species. The bacterial profile of each biofilm is unique, and thus requires early detection and identification to develop a customized treatment plan that combines different therapies most effective on the particular biofilm.
Using the Host Immune System Against Itself
The EPS Matrix - A Mechanical Barrier and Source of Antibiotic Resistance
Pathogenic Synergy in Polymicrobial Biofilms
Methods of Identification and Treatment
Mechanical Debridement
Antibiotic Resistance
Quorum Sensing
Biofilm Disrupting Agents
Section 4
Conclusion
References
- ↑ Assessment Technologies Institute of Nursing Education. “Wound Care: The anatomy and physiology of wound healing.”
- ↑ Dowd, Scot E., Randall D. Wolcott, Yan Sun, Trevor McKeehan, Ethan Smith, and Daniel Rhoads. “Polymicrobial Nature of Chronic Diabetic Foot Ulcer Biofilm Infections Determined Using Bacterial Tag Encoded FLX Amplicon Pyrosequencing (bTEFAP).” 2008. PLoS ONE 3(10): e3326. DOI: 10.1371/journal.pone.0003326.
- ↑ Paddock, Catharine. “Bacteria living on skin may affect how wounds heal.” May 2, 2014. Medical News Today.
- ↑ Watters, Chase, Katrina DeLeon, Urvish Trivedi, John A. Griswold, Mark Lyte, Ken J. Hampel, Matthew J. Wargo, and Kendra P. Rumbaugh. “Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice.” 2013. Medical Microbiology and Immunology 202: 131-141. DOI: 10.1007/s00430-012-0277-7.
- ↑ “IDF Diabetes Atlas Executive Summary.” 7th edition. 2015. International Diabetes Federation.
- ↑ Attinger, Christopher, and Randy Wolcott. “Clinically Addressing Biofilm in Chronic Wounds.” 2011. Advances in Wound Care, 1(3): 127-132. DOI: 10.1089/wound.2011.0333.
- ↑ Watters, Chase, Jake A. Everett, Cecily Haley, Allie Clinton, and Kendra P. Rumbaugh. “Insulin Treatment Modulates the Host Immune System To Enhance Pseudomonas aeruginosa Wound Biofilms.” 2014. Infection and Immunity 82(1): 92-100. DOI: 10.1128/IAI.00651-13.
- ↑ Slonczewski & Foster (2014) Microbiology: An Evolving Science; Chapter 4 Bacterial Culture, Growth, and Development.
- ↑ Ventolini, Gary. “Vaginal Lactobacillus: biofilm formation in vivo – clinical implications.” 2015. International Journal of Women’s Health 7: 243-247.
- ↑ Donlan, Rodney M. “Biofilm Elimination on Intravascular Catheters: Important Considerations for the Infectious Disease Practitioner.” 2011. Clinical Infectious Diseases 52(8): 1038-1045. DOI: 10.1093/cid/cir077.
- ↑ Watters, Chase, Katrina DeLeon, Urvish Trivedi, John A. Griswold, Mark Lyte, Ken J. Hampel, Matthew J. Wargo, and Kendra P. Rumbaugh. “Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice.” 2013. Medical Microbiology and Immunology 202: 131-141. DOI: 10.1007/s00430-012-0277-7.
- ↑ Bertesteanu, Serban, Stefanos Triaridis, Milan Stankovic, Veronica Lazar, Mariana Carmen Chifiriuc, Mihaela Vlad, and Raluca Grigore. “Polymicrobial wound infections: Pathophysiology and current therapeutic approaches.” 2014. International Journal of Pharmaceutics 463: 119-126. DOI: 10.1016/j.ijpharm.2013.12.012.
- ↑ Duplantier, Allen J. and Monique L. Van Hoek. “The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds.” 2013. Frontiers in Immunology 4(143): 1-14. DOI: 10.3389/fimmu.2013.00143.
- ↑ Joo, Hwang-Soo and Michael Otto. “Molecular basis of In Vivo Biofilm Formation by Bacterial Pathogens.” 2012. Chemistry & Biology 19: 1503-1513. DOI: 10.1016/j.chembiol/2012.10.022.
- ↑ Kirketerp-Moller, Karen Zulkowski, and Garth James. “Chronic Wound Colonization, Infection, and Biofilms.” 2011. Biofilm Infections (Chapter 2). DOI: 10.1007/978-1-4419-6084-9_2.
- ↑ Percival, Steven L., and Philip G. Bowler. “Biofilms and Their Potential Role in Wound Healing.” 2004. Wounds 16(7).
- ↑ Percival, Steven L., and Philip G. Bowler. “Understanding the effects of bacterial communities and biofilms on wound healing.” 2004. World Wide Wounds.
- ↑ Tuttle, Marie S., Eliot Mostow, Pranab Mukherjee, Fen Z. Hu, Rachael melton-Kreft, Garth D. Ehrlich, Scot E. Dowd, and Mohmoud A. Ghannoum. “Characterization of Bacterial Communities in Venous Insufficiency Wounds by Use of Conventional Culture and Molecular Diagnostic Methods.” 2011. Journal of Clinical Microbiology 49(11): 3812-3819. DOI: 10.1128/JCM.00847-11.
- ↑ Wolcott, R., J.W. Costerton, D. Raoult, and S.J. Culter. “The polymicrobial nature of biofilm infection.” 2013. Clinical Microbiology and Infection 19(2): 107-112. DOI: 10.1111/j.1469-0691.2012.04001.x
- ↑ Zhao, Ge, Marcia L. Usui, Soyeon I. Lippman, Garth A. James, Philip S. Stewart, Philip Fleckman, and John E. Olerud. “Biofilms and Inflammation in Chronic Wounds.” 2013. Advances in Wound Care 2(7): 389-399. DOI: 10.1089/wound.2012.0381.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.