Modern Treatments for Leprosy: Difference between revisions
| Line 19: | Line 19: | ||
==Signs and Symptoms== | ==Signs and Symptoms== | ||
Symptoms of Hansen’s disease can take many years to materialize, often anywhere from two to ten years, due to the slow-growing nature of the bacteria. The majority of the symptoms affect the epidermis (skin), nerve cells and the mucus membranes lining bodily cavities, such as the lining of the throat and the nasal canals. Most symptoms are painful and can leave the afflicted person disabled if treatment is provided too late. | Symptoms of Hansen’s disease can take many years to materialize, often anywhere from two to ten years, due to the slow-growing nature of the bacteria. The majority of the symptoms affect the epidermis (skin), nerve cells and the mucus membranes lining bodily cavities, such as the lining of the throat and the nasal canals [4][5]. Most symptoms are painful and can leave the afflicted person disabled if treatment is provided too late. | ||
Skin lesions can appear that may in time become faded or discolored. Skin can become thick, stiff or dry and numbness in those affected areas of the skin may follow. Growths on the skin may appear, such as the characteristic boils seen in many patients which may then rupture. As a result of the fact that the infection affects the nerves, severe pain can occur as well as ocular nerve issues which can in turn lead to permanent damage that results in partial or total loss of eyesight in patients. Other problems with the nerves such as muscle weakness or paralysis, particularly in extremities, for instance the hands or feet, are common as well as enlarged nerves often around the knees or elbows. Ulcers on the soles of the afflicted persons feet can also be an issue. As the disease also affects mucous membranes, less serious symptoms such as nosebleeds, sinus infections and stuffy nasal canals can occur. | The symptoms of the disease often manifest in the following ways. Skin lesions can appear that may in time become faded or discolored. Skin can become thick, stiff or dry and numbness in those affected areas of the skin may follow. Growths on the skin may appear, such as the characteristic boils seen in many patients which may then rupture. As a result of the fact that the infection affects the nerves, severe pain can occur as well as ocular nerve issues which can in turn lead to permanent damage that results in partial or total loss of eyesight in patients. Other problems with the nerves such as muscle weakness or paralysis, particularly in extremities, for instance the hands or feet, are common as well as enlarged nerves often around the knees or elbows. Ulcers on the soles of the afflicted persons feet can also be an issue. As the disease also affects mucous membranes, less serious symptoms such as nosebleeds, sinus infections and stuffy nasal canals can occur. Many of these symptoms are caused by damage to the nerves. | ||
The effect of Hansen’s disease on the nerves is by far the most concerning aspect of the disease. Loss of feeling and sensation is particularly concerning because nerve damage can become a perpetual issue and leave an afflicted individual permanently disabled. Due to the loss of sensation and feeling, injuries may be disregarded. Untreated wounds can lead to infection and decrease the immune system’s ability to fight against the pathogen [4]. | The effect of Hansen’s disease on the nerves is by far the most concerning aspect of the disease. Loss of feeling and sensation is particularly concerning because nerve damage can become a perpetual issue and leave an afflicted individual permanently disabled. Due to the loss of sensation and feeling, injuries may be disregarded. Untreated wounds can lead to infection and decrease the immune system’s ability to fight against the pathogen [4]. | ||
==Multi Drug therapy== | ==Multi Drug therapy== | ||
Revision as of 16:06, 8 May 2015
History of Leprosy
by Alexandra Schaal
The disease of leprosy, also known as Hansen’s disease, was believed to have originated in Asia. The earliest records of the disease in Europe appeared after Alexander the Great’s army returned from India and a second time with the return of Pompeii’s troops from Asia Minor. A document dating back to 600 B.C.E. from the Indian subcontinent describes a disease with symptoms consistent with those of leprosy. However, now the first believed reference to leprosy was recorded on Egyptian papyrus and can be dated back to 1550 B.C.E. [2] Since then the disease has spread across the globe. Today, leprosy is a treatable disease and with a much lower rate of incidence. Nonetheless, it is estimated that between one and two million people worldwide suffer from visible and irreversible disabilities left in the wake of the disease. Leprosy can be cured and transmission prevented through antibacterial treatments. However, in poorer and more remote parts of the world, including regions in in Asia, Africa, and South America, afflicted persons had less access to and fewer resources with which to obtain these treatments. This in turn increased incidence and prevalence of the disease. Historically individuals infected with were cast out of society and poorly treated. Transmission of leprosy and the disease itself, were not well understood resulting in the ostracization and shunning of afflicted persons. Throughout varying parts of the world the disease was considered a curse or punishment from God [1]. The disease is much better understood now and afflicted individuals are given help they require [2].

A scientific understanding of leprosy began with Dr. Gerhard H.A. Hansen, after whom the disease is named. He identified the bacterium Mycobacterium leprae in 1873 and proved that the disease was of biological origin. The first breakthrough in medication to treat leprosy happened in the 1940’s with the introduction of the antibacterial drug dapsone. Since then more effective drugs have been introduced [2][1]. Until 2008 the bacterium Mycobacterium leprae was the only known causative agent of Hansen’s disease, when a new species of closely related bacterium, Mycobacterium lepromatosis , was discovered to cause DLL (diffuse lepromatous leprosy). This strain of Hansen’s disease is endemic to Mexico and while less studied than the other strain, it appears to have symptoms different from those caused by Mycobacterium leprae [3]. Since the discovery of the biological cause of the disease, measures have been taken by various organizations to prevent the further spread of the disease [2].
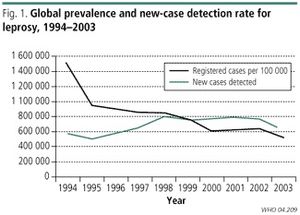
The World Health Assembly (WHA) initiated a program to eliminate Hansen’s disease as a public-health concern in 1991. They strived to accomplish this resolution by the end of the year 2000. The ultimate goal of this program was to reduce the prevalence rate of Hansen’s disease to less than one afflicted person per ten thousand people on a global scale. The goal of global elimination of the disease, by means of the prevalence rate of the disease, was accomplished by the set forth deadline. Since 1995, Multidrug Therapy has been distributed free to all persons afflicted by Hansen’s disease. This free distribution of medication is made possible with the assistance of the World Health Organisation (WHO). The decrease in the prevalence rate of the disease worldwide has been striking since the institution of the aforementioned public-health initiative, although Hansen’s disease is still endemic some developing countries, primarily in countries situated near the equator with tropical climates. As many as sixteen million symptomatic individuals are believed to have been cured of the disease over the past twenty years. Over the duration of that time the prevalence rate of Hansen’s disease is thought to have decreased by at least 90%. In 1985, before the public-health initiative against the disease was instigated, Hansen’s disease was considered a public-health concern in a total of 122 countries globally. Since then the disease has been eliminated in a total of 119 of those countries in which it was considered a public-health issue. However in more recent years there have been many reports of new cases. In 2012, official reports stated that over 230,000 new cases of Hansen’s disease were reported from 115 countries over the globe. Sixteen of these countries accounted for around 95% of the reported incidents of the disease. Some of the countries in which Hansen’s disease is more prevalent and a greater health concern than elsewhere in the world include India , China, Brazil and the Philippines. In 2009 it was recorded in the National Hansen's Disease Registry that there were 213 new cases of the disease reported in the United States. Between 150 and 250 newly diagnosed cases of Hansen’s are reported each year in the United States, most of these cases arising in individuals who had immigrated to the United States. The reason that the total elimination of leprosy is near impossible is due to the fact that Hansen’s is not an exclusively human pathogen. Several species of animals, such as armadillos and including some species of apes, such as chimpanzees, carry the bacterial agent that causes the disease, thus creating the opportunity for the pathogen to jump from one species to humans [2].
Signs and Symptoms
Symptoms of Hansen’s disease can take many years to materialize, often anywhere from two to ten years, due to the slow-growing nature of the bacteria. The majority of the symptoms affect the epidermis (skin), nerve cells and the mucus membranes lining bodily cavities, such as the lining of the throat and the nasal canals [4][5]. Most symptoms are painful and can leave the afflicted person disabled if treatment is provided too late.
The symptoms of the disease often manifest in the following ways. Skin lesions can appear that may in time become faded or discolored. Skin can become thick, stiff or dry and numbness in those affected areas of the skin may follow. Growths on the skin may appear, such as the characteristic boils seen in many patients which may then rupture. As a result of the fact that the infection affects the nerves, severe pain can occur as well as ocular nerve issues which can in turn lead to permanent damage that results in partial or total loss of eyesight in patients. Other problems with the nerves such as muscle weakness or paralysis, particularly in extremities, for instance the hands or feet, are common as well as enlarged nerves often around the knees or elbows. Ulcers on the soles of the afflicted persons feet can also be an issue. As the disease also affects mucous membranes, less serious symptoms such as nosebleeds, sinus infections and stuffy nasal canals can occur. Many of these symptoms are caused by damage to the nerves.
The effect of Hansen’s disease on the nerves is by far the most concerning aspect of the disease. Loss of feeling and sensation is particularly concerning because nerve damage can become a perpetual issue and leave an afflicted individual permanently disabled. Due to the loss of sensation and feeling, injuries may be disregarded. Untreated wounds can lead to infection and decrease the immune system’s ability to fight against the pathogen [4].
Multi Drug therapy
As there are multiple strains of Hansen’s disease there are three major combinations of drugs used to treat varying strains. Rifampicin is used solely in combination with dapsone in order to treat tuberculoid cases of Paucibacillary (PB) leprosy. Dapsone and rifampicin are also used in combination with clofazimine in order to treat lepromatous cases of Multibacillary (MB) leprosy. Recently a single dose treatment combining the drugs ofloxacin, rifampicin, and minocycline has been used as a treatment for single lesion paucibacillary leprosy. Dapsone was used as a monotherapy drug for a time until resistant strains of the disease evolved. Now, use of any one drug as a monotherapy treated is prohibited in order to attempt to prevent the mutation multi-drug resistant strains [6].
Dapsone
Dapsone a sulfone drug, belonging to a class of anti-infective drugs, is bacteriostatic or weakly bactericidal against Mycobacterium leprae [5][6]. It was the first drug used to treat Hansen’s disease. The exact mechanism of interaction between the bacteria and the drug is unknown. Dapsone is primarily used to treat Hansen’s disease. However it is also used to treat dermatitis herpetiformis, an infection of the skin. For the most part dapsone is considered a relatively safe drug however, in rarer cases, dapsone is believed to have caused certain serious and potentially fatal complications involving function of the liver and blood. The most common adverse effect is hemolysis which can also occur in patients that have a G6PD deficiency and those without a deficiency. Often a small decrease in hemoglobin is demonstrated, as well as an increase in reticulocytes and methemoglobin and shortened red blood cell lifespan. These effects are often heightened in patients with a G6PD deficiency. Rarely nerval issues may occur in patients, but may be difficult to identify in patients with Hansen’s disease due to the nature of the infection and the symptoms exhibited. Dapsone is given orally, and is nearly completely absorbed at a rapid rate. The drug is excreted through the urinary tract. Excretion is slow, resulting in near constant blood levels when dapsone is taken regularly [6]. For Paucibacillary Leprosy there is a six month course of treatment. In adults the usual dose is 100 mg daily and for children the dose is reduced to 50 mg daily. For Multibacillary Leprosy there is a twelve month regimen for treatment with identical dosages, in both children and adults, as Paucibacillary Leprosy. Dosages can vary with weight, age and other factors [5]. Despite the low incidence of side effects, dapsone or sulfone syndrome (DDS) can occur. The rate of occurrence of DDS has increased and is thought to be due to the high starting dose of the treatment (100 mg) and possibly due to a genetic susceptibility to dapsone and the other drugs used in the multi-drug therapy in some populations [7].
Rifampicin
Rifampicin, branded as Rifadin, is derived from rifamycin SV and is a semisynthetic antibiotic. Rifampicin is also used to treat tuberculosis and meningococcal carriers. Rifampicin is known to cause some gastrointestinal issues including but not limited to nausea, vomiting, and diarrhea. Abnormalities in the liver have been observed in patients taking the drug over a longer duration of time. When taken in high dose intermittent therapies, as is the case in some leprosy treatments, thrombocytopenia (irregular platelet count) can become an issue, resulting in poor blood flow and difficulty in forming proper blood clots. Decreased hemoglobin can also occur. These adverse effects can be reversed through discontinuation of the treatment and tend to not occur in patients taking lower daily doses. Cerebral hemorrhage and fatalities have been reported after the resumption or discontinuation of administration of the drug after adverse effects had been observed in the patients. It is believed that some damage to the nervous system can occur, however due to the symptoms of Hansen’s disease it is quite impossible to distinguish effects of the drug from ordinary symptoms of the disease. Ocular issues are also known to have occurred in patients, but as with nerve issues Hansen’s is known to cause cause ocular issues as well, thus making it vastly difficult to distinguish symptoms of the disease from side effects of the drug. Rifampicin is also believed to disrupt some aspects of the endocrine system resulting in disturbances in the menstrual cycle of female patients. Some skin irritations can occur and would likely go unnoticed by persons afflicted with Hansen’s disease. Rifampicin is generally administered orally and absorbed through the gastrointestinal tract. Rifampicin is excreted through bile. Once absorbed the drug becomes present in most bodily fluids and organ tissues, including cerebrospinal fluid, possibly explaining the drug’s ability to affect the central nervous system and the cerebral hemorrhages and fatalities that have been reported due to the resumption or discontinuation of administration of the drug after adverse effects had been observed in the patients. Absorption can be affected by the consumption of food and other medication. Rifampicin can also be taken intravenously under the supervision of a physician. Rifampicin interacts with bacterial DNA-dependent RNA polymerase but does not inhibit the human enzyme. Rifampicin not only helps to slow the growth of the pathogen but also prevents the spread of the disease to other individuals [8]. For the treatment of paucibacillary leprosy under a six month regimen of treatment, a single dose is given under supervision of a physician. In adults the monthly dose is 600 mg. In children the dose is decreased to 450 mg each month. In the case of the twelve month regimen in order to treat cases of multibacillary leprosy, rifampicin is administered monthly under the direct supervision of a physician and the dosages are the same those for the six month paucibacillary leprosy treatment regimen for both children and in adults.However unlike in the treatment of paucibacillary leprosy 50 mg of rifampicin is also administered daily to adults and every other day to children, the dosage being the same in both children and adults. In single lesion paucibacillary leprosy cases rifampicin is combined with two other drugs to create a single dose treatment. The dosage for adults 600 mg and in children the dosage is reduced to 300 mg. This treatment is not recommended for pregnant women and for children under the age of five. Dosages can vary with weight, age and other factors [5].
Clofazimine
Clofazimine, also known as lamprene, is used exclusively as an anti-leprosy agent and is administered orally. Clofazimine is known to cause skin discoloration in nearly all patients, changing the colorization of the skin from pink to a brownish-black in 75% to 100% of patients within the first few weeks of treatment. Dryness and scale-like formations on the skin occur in a tenth to third of all patients. Rashes may also occur, however due to skin conditions arising from the infection this adverse effect may be overlooked by afflicted persons. Clofazimine is also known to cause gastrointestinal issues including, but not limited to, abdominal pain, diarrhea, nausea, and vomiting. One or more of these symptoms occur in 40% to 50% of the population. Crystal deposits can form in the eyes of patients causing conjunctival and corneal pigmentation. These crystal deposits can also cause irritation, dryness, itching and burning. However, these crystal deposits do not cause any form of ocular nerve damage and the pigmentation can be reversed through the discontinuation of treatment with clofazimine. Discoloration can also occur in the urine, fecal matter, and sweat. Clofazimine is also believed to cause elevated blood sugar levels so those with preexisting conditions associated with elevated blood sugar levels may be at risk. Clofazimine exerts a slow bactericidal effect on Mycobacterium leprae . Clofazimine inhibits mycobacterial growth by preferentially binding to mycobacterial DNA. Clofazimine also has anti-inflammatory properties which is beneficial to person’s afflicted with Hansen's disease because of the way in which the pathogen affects the mucous membranes. As of now, the exact mechanism of action is unknown. Absorption of the drug varies from patient to patient ranging from 45% to 62% under oral administration. Clofazimine is not water soluble, but is highly lipid soluble. Clofazimine remains in the human body for an extended period of time. The half-life of clofazimine taken orally for an extended time period is believed to be astonishingly high, at least 70 days. Clofazimine is believed to be partially excreted through bile and also through mucus from the lungs and sweat. Clofazimine crystals form in many parts of the body throughout the duration of treatment, including but not limited to the lymph nodes, adrenal glands, liver, spleen, small intestine, bones, muscle tissue and the skin [9]. Clofazimine is used in the twelve month treatment regimen of multibacillary leprosy in which it is administered monthly under the supervision of a physician. The dosage for adults being 300 mg. For children the dosage is decreased to 150 mg. Dosages can vary with weight, age and other factors [5].
Ofloxacin and Minocycline
Ofloxacin, also known as floxin, belongs to a group of antibiotics called fluoroquinolones. The exact mechanism of action is unknown but, it is believed that the drug prevents bacterial DNA from unwinding thus preventing replication of the bacterial DNA while not inhibiting human DNA replication and repair. Ofloxacin is known to have adverse effects on the cardiovascular and gastrointestinal systems as a whole, as well as having adverse effects on other systems, including but not limited to, the muscular, reproductive, and nervous systems [10]. Ofloxacin is used in the single dose treatment of single lesion paucibacillary leprosy. A dose of 400 mg is given to adults and a dose of 200 mg to children. In accompaniment, the drug minocycline is given in dosages of 100 mg in adults and 50 mg is children. Dosages can vary with weight, age and other factors [5].
References
[1] "Leprosy (Hansen's Disease)." History of Leprosy. NIAID, 08 Feb. 2011. Web. 20 Apr. 2015. <http://www.niaid.nih.gov/topics/leprosy/understanding/Pages/history.aspx>.
[2] Doerr, Steven. "Leprosy: Get Facts on the Symptoms and History." EMedicineHealth. EMedicineHealth, 19 Sept. 2014. Web. 20 Apr. 2015. <http://www.emedicinehealth.com/leprosy/article_em.htm>.
[3] Han, Xiang Y., Kurt Clement Sizer, Jesús S. Velarde-Félix, Luis O. Frias-Castro, and Francisco Vargas-Ocampo. "The Leprosy Agents Mycobacterium Lepromatosis and Mycobacterium Leprae in Mexico." International Journal of Dermatology 51.8 (2012): 952-59. PubMed. Web. 20 Apr. 2015.
[4] "Hansen's Disease (Leprosy)." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 29 Apr. 2013. Web. 20 Apr. 2015. <http://www.cdc.gov/leprosy/symptoms/>.
[5] "WHO Model Prescribing Information: Drugs Used in Leprosy: Treatment of Leprosy." WHO Model Prescribing Information: Drugs Used in Leprosy: Treatment of Leprosy. Human Info NGO, 11 Apr. 2015. Web. 20 Apr. 2015. <http://apps.who.int/medicinedocs/en/d/Jh2988e/5.html>.
[6] "Dapsone (Dapsone) Drug Information: Description, User Reviews, Drug Side Effects, Interactions - Prescribing Information at RxList." RxList. RxList, 19 Mar. 2014. Web. 20 Apr. 2015. <http://www.rxlist.com/dapsone-drug.htm>.
[7] Reeve, P.A., J. Ala, and J.J. Hall. "Dapsone Syndrome in Vanuatu: A High Incidence during Multidrug Treatment (MDT) of Leprosy." The Journal of Tropical Medicine and Hygiene 95.4 (1992): 266-70. Europe PubMed Central. Web. 20 Apr. 2015.
[8] "Rifadin (Rifampin) Drug Information: Description, User Reviews, Drug Side Effects, Interactions - Prescribing Information at RxList." RxList. RxList, 18 Mar. 2013. Web. 20 Apr. 2015. <http://www.rxlist.com/rifadin-drug.htm>.
[9] "Lamprene (Clofazimine) Drug Information: Description, User Reviews, Drug Side Effects, Interactions - Prescribing Information at RxList." RxList. RxList, 11 May 2008. Web. 20 Apr. 2015. <http://www.rxlist.com/lamprene-drug.htm>.
[10] "Floxin (Ofloxacin) Drug Information: Description, User Reviews, Drug Side Effects, Interactions - Prescribing Information at RxList." RxList. RxList, 15 Apr. 2011. Web. 20 Apr. 2015. <http://www.rxlist.com/floxin-drug.htm>.
