Moraxella catarrhalis pathogenesis: Difference between revisions
(Created page with "Category:Pages edited by students of Tyrrell Conway at the University of Oklahoma {{curated}} [[Image:OULOGOBIANCO.JPEG|thumb|135px|left|University of Oklahoma Study Abro...") |
No edit summary |
||
| Line 20: | Line 20: | ||
==Description== | ==Description== | ||
<i>Moraxella catarrhalis</i> is a gram negative, diplococcus, aerobic infectious pathogen that is a common cause of upper | <i>Moraxella catarrhalis</i> is a gram negative, diplococcus, aerobic infectious pathogen that is a common cause of upper respiratory, middle ear, and eye infections. It is resistant to many ß-lactam drugs such as penicillin, amoxicillin, and ampicillin. 'M. catarrhalis' can be found commensally in the respiratory tract of some people, living without infections symptoms or promoting host immune response. In adults, the rate of colonization seems to be around 3% of healthy adult population [[#References|[1]]]. This rate seems to be much higher in children, especially in infants in their first year of life. Depending on factors such as socioeconomic background, climate, and location, colonization rates have varied anywhere from 28% in Sweden, to 100% of Australian Aborigines [[#References|[2,3]]]. It is estimated that <i>M. catarrhalis</i> causes nearly four million cases of middle ear infection a year which, while not particularly life threatening, places a burden of nearly two billion dollars on the US health care system [[#References|[4]]]. There are two distinctive strains of <i>M. catarrhalis</i>: serosensitive, which seems to be less virulent, and seroresistant, which has complement resistance and adherence mechanisms that make it particularly virulent [[#References|[5]]]. | ||
==Pathogenesis== | ==Pathogenesis== | ||
| Line 30: | Line 30: | ||
The incubation period of <i>M. catarrhalis</i> is not well understood. This is because it is a commensal organism in many persons and can live asymptomatically for a long period of time. However, this suggests that it is an opportunistic pathogen because it will show symptoms in those who have undergone significant stress to their nasopharynyx such as patients with COPD. | The incubation period of <i>M. catarrhalis</i> is not well understood. This is because it is a commensal organism in many persons and can live asymptomatically for a long period of time. However, this suggests that it is an opportunistic pathogen because it will show symptoms in those who have undergone significant stress to their nasopharynyx such as patients with COPD. | ||
<br> | <br> | ||
It is believed that bacterial stress such as heat shock could cause activation of pathogenic mechanisms within <i>M. catarrhalis</i> [[#References|[7]]]. This type of reaction is well documented in other organisms like <i>E. Coli</i>, <i>B. Subtilis</i>, and <i>S. aureus</i>. This may account for the prevalence of the disease during the colder seasons such as fall and winter. It has been found that | It is believed that bacterial stress such as heat shock could cause activation of pathogenic mechanisms within <i>M. catarrhalis</i> [[#References|[7]]]. This type of reaction is well documented in other organisms like <i>E. Coli</i>, <i>B. Subtilis</i>, and <i>S. aureus</i>. This may account for the prevalence of the disease during the colder seasons such as fall and winter. It has been found that prolonged exposure to conditions at 26°C increases the abundance of mRNA transripts coding for adhesion molecules associated with the pathogenicity of <i>M. catarrhalis</i> [[#References|[8]]]. | ||
===Epidemiology=== | ===Epidemiology=== | ||
<i>M. catarrhalis</i> can be a commensal organism found in the respiratory tract of young children and infants, and in a small percentage of adults depending on factors such as location, age and health. If symptomatic, it commonly presents as otitis media, or infection of the middle ear, in children and as an upper respiratory infection in adults. Because of widely varying colonization rates | <i>M. catarrhalis</i> can be a commensal organism found in the respiratory tract of young children and infants, and in a small percentage of adults depending on factors such as location, age and health. If symptomatic, it commonly presents as otitis media, or infection of the middle ear, in children and as an upper respiratory infection in adults. Because of widely varying colonization rates throughout studies, more information must be gathered to understand these processes [[#References|[9]]]. | ||
===Virulence Factors=== | ===Virulence Factors=== | ||
====Antibiotic Resistance==== | ====Antibiotic Resistance==== | ||
Since the discovery of a β-lactamase-positive strain of <i>M. catarrhalis</i>, the resistance of the species to β-lactam drugs has increased at a rate much greater than other bacterial species. More than 90% of known isolates are β-lactamase-positive [[#References|[10]]]. | [[Image:BRO Beta lactamase.jpeg|thumb|150px|right|Electron micrograph of <i>M. catarrhalis</i> secreting BRO β-lactamase after sucrose shock. From: [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC94001/]]] | ||
Since the discovery of a β-lactamase-positive strain of <i>M. catarrhalis</i>, the resistance of the species to β-lactam drugs has increased at a rate much greater than other bacterial species. More than 90% of known isolates are β-lactamase-positive [[#References|[10]]]. The origins of this structure are unknown, as it seems to be very unique to the species, existing at two forms: BRO-1 and BRO-2. Unlike other gram negative bacteria, the enzyme is lipidated and membrane associated. It is possible that this might have evolved from a similar enzyme found in gram positive bacteria. The evidence of this is the distinctive G+C content of the BRO region compared to the rest of the genome [[#References|[11]]]. BRO-1 expressive strains seem to have a greater antibiotic resistance than BRO-2 strains. This does not result from a difference in enzymatic activity, but from variance in expression [[#References|[12]]]. | |||
====Outer Membrane Proteins==== | |||
=====<font color=black>CopB</font>===== | |||
CopB is a surface protein expressed that is believed to be involved in acquisition of iron from host lactoferrin or transferrin molecules. Studies have shown that CopB is expressed much more readily under conditions of low iron and that CopB will bind with human lactoferrin molecules when they are under heavy load [[#References|[13,14]]]. Anti-CopB antibodies have been found in blood serum of patients previously infected and have been studied for potential methods of enhancing clearance. Although <i>M. catarrhalis</i> is strictly a human pathogen and results are therefore inconclusive, animal models have shown increased clearance in CopB knockout infection of rats [[#References|[15]]]. | |||
=====<font color=black>OMP CD</font>===== | |||
The function of OMP CD is not very well understood. It shares some characteristics with the OprF porin Pueudomonas, however its exact function has not been proven. Like CopB, studies have been conducted to examine it as a potential basis for vaccines. Again, the certainty of animal models is uncertain, but mice that have been immunized with CD have shown much better bacterial clearance rates than controls [[#References|[16]]]. | |||
==Clinical Features== | ==Clinical Features== | ||
| Line 74: | Line 81: | ||
10. Felmingham D, Washington J: Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens--findings of the Alexander Project 1992-1996. <i>J. Chemother.</i> 1999. 11:5–21. | 10. Felmingham D, Washington J: Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens--findings of the Alexander Project 1992-1996. <i>J. Chemother.</i> 1999. 11:5–21. | ||
11. Bootsma HJ, Aerts PC, Posthuma G, Harmsen T, Verhoef J, van Dijk H, Mooi FR: <i>Moraxella (Branhamella) catarrhalis</i> BRO β-Lactamase: a Lipoprotein of Gram-Positive Origin? <i>J Bacteriol.</i> 1999. 181:16:5090–5093. | |||
12. Wallace Jr RJ, Nash DR, Steingrube VA: Antibiotic susceptibilities and drug resistance in <i>Moraxella (Branhamella) catarrhalis</i>. <i>Am. J. M</i> 1990. 88:46S–50S. | |||
13. Aebi C, Stone B, Beucher M, Cope LD, Maciver I, Thomas SE, McCracken Jr GH, Sparling PF, Hansen EJ: Expression of the CopB outer membrane protein by <i>Moraxella catarrhalis</i> is regulated by iron and affects iron acquisition from transferrin and lactoferrin. <i>Infect. Immun.</i> 1996. 64:2024–2030. | |||
14. R.A. Bonnah, R.H. Yu, H. Wong, A.B. Schryvers: Biochemical and immunological properties of lactoferrin binding proteins from <i>Moraxella (Branhamella) catarrhalis</i>. <i>Microb. Pathog.</i> 1998. 24:89–100. | |||
15. Helminen ME, MacIver I, Latimer JL, Cope LD, McCracken Jr GH, Hansen EJ: A major outer membrane protein of <i>Moraxella catarrhalis</i> is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. <i>Infect. Immun.</i> 1993. 61:2003–2010. | |||
16. Murphy TF, Kyd JM, John A, Kirkham C, Cripps AW: Enhancement of pulmonary clearance of <i>Moraxella (Branhamella) catarrhalis</i> following immunization with outer membrane protein CD in a mouse model. <i>J. Infect. Dis.</i> 1998. 178:1667–1675. | |||
Created by Robertson Bootes Beasley, student of Tyrrell Conway at the University of Oklahoma.<br> | Created by Robertson Bootes Beasley, student of Tyrrell Conway at the University of Oklahoma.<br> | ||
Revision as of 13:52, 29 July 2014

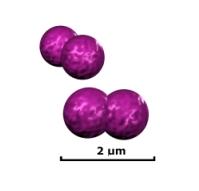
Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Proteobacteria
| Class = Gammaproteobacteria
| Order = Pseudomonadales
| Family = Moraxellaceae
| Genus = 'Moraxella'
| Species = 'M. catarrhalis'
Description
Moraxella catarrhalis is a gram negative, diplococcus, aerobic infectious pathogen that is a common cause of upper respiratory, middle ear, and eye infections. It is resistant to many ß-lactam drugs such as penicillin, amoxicillin, and ampicillin. 'M. catarrhalis' can be found commensally in the respiratory tract of some people, living without infections symptoms or promoting host immune response. In adults, the rate of colonization seems to be around 3% of healthy adult population [1]. This rate seems to be much higher in children, especially in infants in their first year of life. Depending on factors such as socioeconomic background, climate, and location, colonization rates have varied anywhere from 28% in Sweden, to 100% of Australian Aborigines [2,3]. It is estimated that M. catarrhalis causes nearly four million cases of middle ear infection a year which, while not particularly life threatening, places a burden of nearly two billion dollars on the US health care system [4]. There are two distinctive strains of M. catarrhalis: serosensitive, which seems to be less virulent, and seroresistant, which has complement resistance and adherence mechanisms that make it particularly virulent [5].
Pathogenesis
Transmission/Reservoirs
Because of its ability to live commensally, the spread of M. catarrhalis is generally from person to person in a hospital setting, or nosocomially [6]. Infections seem to occur more in persons with complications of the respiratory system such as COPD or infants with underdeveloped immune systems. Additionally, if a patient is diagnosed with symptoms of an infection, it can be difficult to determine if M. catarrhalis is the cause due to its ability to live commensally, and as similar symptoms can arise through infections from S. pneumoniae and H. influenzae.
Incubation/Colonization
The incubation period of M. catarrhalis is not well understood. This is because it is a commensal organism in many persons and can live asymptomatically for a long period of time. However, this suggests that it is an opportunistic pathogen because it will show symptoms in those who have undergone significant stress to their nasopharynyx such as patients with COPD.
It is believed that bacterial stress such as heat shock could cause activation of pathogenic mechanisms within M. catarrhalis [7]. This type of reaction is well documented in other organisms like E. Coli, B. Subtilis, and S. aureus. This may account for the prevalence of the disease during the colder seasons such as fall and winter. It has been found that prolonged exposure to conditions at 26°C increases the abundance of mRNA transripts coding for adhesion molecules associated with the pathogenicity of M. catarrhalis [8].
Epidemiology
M. catarrhalis can be a commensal organism found in the respiratory tract of young children and infants, and in a small percentage of adults depending on factors such as location, age and health. If symptomatic, it commonly presents as otitis media, or infection of the middle ear, in children and as an upper respiratory infection in adults. Because of widely varying colonization rates throughout studies, more information must be gathered to understand these processes [9].
Virulence Factors
Antibiotic Resistance
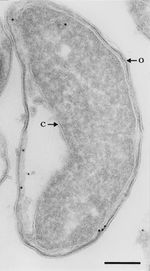
Since the discovery of a β-lactamase-positive strain of M. catarrhalis, the resistance of the species to β-lactam drugs has increased at a rate much greater than other bacterial species. More than 90% of known isolates are β-lactamase-positive [10]. The origins of this structure are unknown, as it seems to be very unique to the species, existing at two forms: BRO-1 and BRO-2. Unlike other gram negative bacteria, the enzyme is lipidated and membrane associated. It is possible that this might have evolved from a similar enzyme found in gram positive bacteria. The evidence of this is the distinctive G+C content of the BRO region compared to the rest of the genome [11]. BRO-1 expressive strains seem to have a greater antibiotic resistance than BRO-2 strains. This does not result from a difference in enzymatic activity, but from variance in expression [12].
Outer Membrane Proteins
CopB
CopB is a surface protein expressed that is believed to be involved in acquisition of iron from host lactoferrin or transferrin molecules. Studies have shown that CopB is expressed much more readily under conditions of low iron and that CopB will bind with human lactoferrin molecules when they are under heavy load [13,14]. Anti-CopB antibodies have been found in blood serum of patients previously infected and have been studied for potential methods of enhancing clearance. Although M. catarrhalis is strictly a human pathogen and results are therefore inconclusive, animal models have shown increased clearance in CopB knockout infection of rats [15].
OMP CD
The function of OMP CD is not very well understood. It shares some characteristics with the OprF porin Pueudomonas, however its exact function has not been proven. Like CopB, studies have been conducted to examine it as a potential basis for vaccines. Again, the certainty of animal models is uncertain, but mice that have been immunized with CD have shown much better bacterial clearance rates than controls [16].
Clinical Features
Diagnosis
Treatment
Prevention
Risk Factors
Host Immune Response
References
1. Jousimies-Somer HR, Savolainen S, Ylikoski JS: Comparison of the nasal bacterial floras in two groups of healthy subjects and in patients with acute maxillary sinusitis. J. Clin. Microbiol. 1989. 28:2736–2743.
2. Aniansson G, Alm B, Andersson B, Larsson P, Nylen O, Peterson H, Rigner P, Svanborg M, Svanborg C: Nasopharyngeal colonization during the first year of life. J. Infect. Dis. 1992. 165:S38–S42.
3. Leach AJ, Boswell JB, Asche V, Nienhuys TG, Mathews JD: Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants. Pediatr. Infect. Dis. J. 1994. 13:983–989.
4. Klein JO: Otitis media. Clin. Infect. Dis. 1994. 19:823–833.
5. Wirth T, Morelli G, Kusecek B, van Belkum A, van der Schee C, Meyer A, Achtman M: The rise and spread of a new pathogen: seroresistant Moraxella catarrhalis. Genome Res. 2007. 17:11:1647-56.
6. Richards SJ, Greening AP, Enright MC, Morgan MG, McKenzie H: Outbreak of Moraxella catarrhalis in a respiratory unit. Thorax 1993. 48:91-92.
7. Aebi C: Moraxella catarrhalis - pathogen or commensal? Adv Exp Med Biol. 2011. 697:107-16.
8. Spaniol V, Troller R, Aebi C: Physiologic cold shock increases adherence of Moraxella catarrhalis to and secretion of interleukin 8 in human upper respiratory tract epithelial cells. J Infect Dis. 2009. 200:1593–1601.
9. Karalusa R, Campagnari A: Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes and Infection 2000. 2:5:547-559.
10. Felmingham D, Washington J: Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens--findings of the Alexander Project 1992-1996. J. Chemother. 1999. 11:5–21.
11. Bootsma HJ, Aerts PC, Posthuma G, Harmsen T, Verhoef J, van Dijk H, Mooi FR: Moraxella (Branhamella) catarrhalis BRO β-Lactamase: a Lipoprotein of Gram-Positive Origin? J Bacteriol. 1999. 181:16:5090–5093.
12. Wallace Jr RJ, Nash DR, Steingrube VA: Antibiotic susceptibilities and drug resistance in Moraxella (Branhamella) catarrhalis. Am. J. M 1990. 88:46S–50S.
13. Aebi C, Stone B, Beucher M, Cope LD, Maciver I, Thomas SE, McCracken Jr GH, Sparling PF, Hansen EJ: Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect. Immun. 1996. 64:2024–2030.
14. R.A. Bonnah, R.H. Yu, H. Wong, A.B. Schryvers: Biochemical and immunological properties of lactoferrin binding proteins from Moraxella (Branhamella) catarrhalis. Microb. Pathog. 1998. 24:89–100.
15. Helminen ME, MacIver I, Latimer JL, Cope LD, McCracken Jr GH, Hansen EJ: A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect. Immun. 1993. 61:2003–2010.
16. Murphy TF, Kyd JM, John A, Kirkham C, Cripps AW: Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J. Infect. Dis. 1998. 178:1667–1675.
Created by Robertson Bootes Beasley, student of Tyrrell Conway at the University of Oklahoma.
