Neisseria meningitidis -- Meningitis: Difference between revisions
| Line 27: | Line 27: | ||
===Infectious dose, incubation, and colonization=== | ===Infectious dose, incubation, and colonization=== | ||
The infectious dose of <i>N. meningitidis</i> is unknown, but the transmission of bacteria is highly contagious. On average, the incubation period is 2-4 days but can range from approximately 2-10 days, while invasive meningococcal infections occur within 14 days of acquiring the bacteria. (Canada) Individuals with <i>N. meningitidis</i> infections are most contagious during the period of 3 days preceding initial symptom presentation and continue to remain infectious while meningococci persists in nasalpharynx discharge. (mening 1) Humans serve as the sole natural reservoir for <i>N. meningitidis</i>, and 10% to 35% of adults and adolescents are asymptomatic carriers, with the majority of bacteria carried as nonpathogenic strains. (mening) (cs118) Carriers of meningococci have the bacteria as a commensal microorganism residing in the respiratory tract and nasopharynx mucosa. It is the penetration of these colonized bacteria into the mucosal membrane and subsequently into the bloodstream that results in various forms of illness. (carriage state) | The infectious dose of <i>N. meningitidis</i> is unknown, but the transmission of bacteria is highly contagious. On average, the incubation period is 2-4 days but can range from approximately 2-10 days, while invasive meningococcal infections occur within 14 days of acquiring the bacteria. (Canada) Individuals with <i>N. meningitidis</i> infections are most contagious during the period of 3 days preceding initial symptom presentation and continue to remain infectious while meningococci persists in nasalpharynx discharge. (mening 1) Humans serve as the sole natural reservoir for <i>N. meningitidis</i>, and 10% to 35% of adults and adolescents are asymptomatic carriers, with the majority of bacteria carried as nonpathogenic strains. (mening) (cs118) Carriers of meningococci have the bacteria as a commensal microorganism residing in the respiratory tract and nasopharynx mucosa. It is the penetration of these colonized bacteria into the mucosal membrane and subsequently into the bloodstream that results in various forms of illness. (carriage state) | ||
===Epidemiology=== | |||
While <i>N. meningitidis</i> is found worldwide, the region of highest incidence is located in sub-Saharan Africa. Referred to as the “meningitis belt”, the frequency of meningococcal disease in this area is several times higher than that of the United States. During the dry season (December-June), the outbreak of sporadic epidemics transpires with as many as 1,000 cases per 100,000 population. Nonepidemic periods have lower rates of occurrence, with approximately 5-10 cases for every 100,000 inhabitants in the region. The outbreaks in the African meningitis belt are usually a result of the serotype A group of <i>N. meningitidis</i>, although minor cases are at times due to serogroups C, X, and W-135. Individuals travelling to areas located within sub-Saharan Africa are at significant risk of contracting disease after extended contact with local inhabitants during the course of an epidemic (CDC) | |||
Outbreaks of meningococcal disease are predominately found in the meningitis belt, but they also appear in the Unitd States. 90% of meningococcal diseases in the United States are a result of serogroups B and C. The average annual rate of invasive diseases caused by <i>N. meningitidis</i> is estimated to be 1.1 cases per 100,000 people or 2,600 cases annually. (PHC mening) Those most at risk for meningococcal disease are toddlers, with rates of incidence estimated to be 46% in children younger than 2 years. Approximately 25% of infections occur in patients older than 30 years. (PHC) | |||
====Morbidity and Mortality==== | |||
The mortality rates of illness caused by <i>N. meningitidis</i> differs depending on the region, country, and age group (CDC manual). Typically, 5% to 10% of patients with meningococcal meningitis die, usually within a period of 24 to 48 hours of initial symptom presentation, despite prompt diagnosis and antibiotic therapy. Without treatment, individuals afflicted with meningitis have a 50% mortality rate. Among the patients that recover from invasive meningococcal diseases, approximately 10% to 20% have permanent sequelae including hearing loss, neurologic damage, or learning disabilities. (WHO) Other complications that occur infrequently include blindness, seizures, myocarditis, pericarditis, ataxia, pneumonia, conjunctivitis, and chronic meningococcemia. (PHC mening) | |||
===Virulence Factors=== | |||
<i>N. meningitidis</i> utilize a variety of virulence factors to aid in its survival and proliferation in the host reservoir. | |||
Capsules: The absence or presence of encapsulated <i>N. meningitidis</i> is highly dependent on the origin of sample. When isolated from carriers, the bacteria may be either capsulate or acapsulate, while samples isolated from cerebrospinal fluid or the bloodstream are consistently capsulate. The presence of capsules in pathogenic <i>N. meningitidis</i>serve to provide resistance against antibody, complement-mediated, or phagocytic destruction by the host immune response. Serogroups B, C, W-135, and Y incorporate sialic acids into the capsule, allowing for evasion by the immune response as sialic acids are also commonly found on several host cell surfaces. A phenomenon identified as capsule switching occurs between the aforementioned serogroups, a process which allows for horizontal exchange of the capsule operon, thereby resulting in anti-capsular antibodies to prove ineffective in eliminating the pathogen. (cs118) | |||
LPS: Endotoxin is a cardinal virulence factor in <i>N. meningitidis</i>, inducing septic shock in patients by triggering pro-inflammatory mediator production. However, the LPS in this species are referred to as lipooligosaccharides (LOS) due to the lack of repeating O-antigens in the polysaccharide structure. Is it the oligosaccharides that contribute to the 12 differing immunotypes of <i>N. meningitidis</i> which are also subject to phase variation via frameshift mutations, allowing the pathogen to circumvent the host immune response. (carriage state) | |||
Adhesion: Several structural and molecular features allow bacteria to adhere to the mucosal surfaces for colonization. <i>N. meningitidis</i> use filaments known as type IV pili located on its surface to adhere to the CD46 membrane coreceptors of host cells. (carriage state) Pili of the Neisseria genus are approximately 6 nm in diameter. In addition to pili, <i>N. meningitidis</i> also express Opa and Opc, two types of proteins found on the outer membrane of the bacteria. Through genetic discrepancy, Opa proteins frequently undergo antigenic variation. Host cells such as epithelial and endothelial cells express a family of receptors known as CEACAM (carcinioembryonic antigen-related cell-adhesion molecule) that bind to Opa proteins during adhesion, while Opc proteins bind to other specific receptors on host endothelial cells. Both types of proteins mediate adhesion and invasion of host cells to facilitate the spread of the pathogen. (cs118) In addition to Opa and Opc proteins, <i>N. meningitidis</i> strains also express and secrete many other proteins that bind to human cells. Amongst these proteins are lactoferrin- and transferrin-binding proteins that also assist the bacteria in binding iron, a fundamental growth factor for colonization and the spread of disease. (cs118) | |||
==Clinical features== | ==Clinical features== | ||
Revision as of 10:20, 27 July 2014

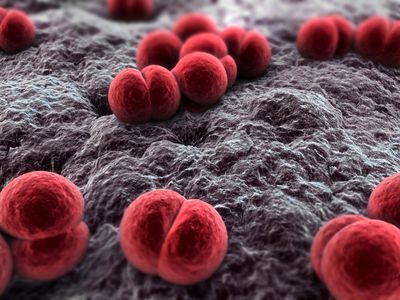
Etiology/Bacteriology
Taxonomy
| Domain = Prokaryote | Phylum = Proteobacteria | Class = Betaproteobacteria | Order = Neisseriales | Family = Neisseriaceae | Genus = Neisseria | species = Neisseria meningitidis
Description
Neisseria meningitidis is an aerobic, Gram-negative diplococcus that causes meningococcal diseases such as meningococcemia and bacterial meningitis. Meningitis arises upon inflammation of the meninges, which consists of the membrane that envelops and protects the central nervous system. N. meningitidis is also commonly known as meningococcus and is carried by approximately 8-25% of the general population in the normal mucosa of the nasopharynx and upper respiratory tract. The varying compositions of the polysaccharide capsule on different strains of N. meningitidis allow the species to be divided into several serogroups, with serogroups A, B, and C as the prominent strains responsible for outbreaks of meningitis in both developed and undeveloped countries. The infection is transmitted to individuals through contact with respiratory secretions or saliva from sneezing, coughing, and talking. Early diagnosis and immediate treatment are vital to the survival of patients assumed to be infected with meningococcal meningitis. Symptoms of the disease are sudden onset and include fever, neck stiffness, and severe headache. (Ref. CDC and Bioquell)
Pathogenesis
Transmission
Meningococci have been categorized into 13 serogroups: A, B, C, D, 29E, H, I, K, L, W-135, X,Y, and Z. Of the 13 serogroups, A, B, C, W135, and Y remain encapsulated, causing more than 90% of the invasive disease across the world. (carriage state) N. meningitidis commonly colonizes in the nasopharynx. As a result, droplets or discharge from the nose or throat that contain N. meningitidis can be transferred to other individuals, resulting in successful transmission of the species. Direct contact with the respiratory droplets can occur through coughing, sneezing, and kissing. (mening 1? CHECK CITATION) Transmission also occurs at higher frequencies in environments where crowding is common, such as prisons, dormitories, and military installations. (PHC mening)
Infectious dose, incubation, and colonization
The infectious dose of N. meningitidis is unknown, but the transmission of bacteria is highly contagious. On average, the incubation period is 2-4 days but can range from approximately 2-10 days, while invasive meningococcal infections occur within 14 days of acquiring the bacteria. (Canada) Individuals with N. meningitidis infections are most contagious during the period of 3 days preceding initial symptom presentation and continue to remain infectious while meningococci persists in nasalpharynx discharge. (mening 1) Humans serve as the sole natural reservoir for N. meningitidis, and 10% to 35% of adults and adolescents are asymptomatic carriers, with the majority of bacteria carried as nonpathogenic strains. (mening) (cs118) Carriers of meningococci have the bacteria as a commensal microorganism residing in the respiratory tract and nasopharynx mucosa. It is the penetration of these colonized bacteria into the mucosal membrane and subsequently into the bloodstream that results in various forms of illness. (carriage state)
Epidemiology
While N. meningitidis is found worldwide, the region of highest incidence is located in sub-Saharan Africa. Referred to as the “meningitis belt”, the frequency of meningococcal disease in this area is several times higher than that of the United States. During the dry season (December-June), the outbreak of sporadic epidemics transpires with as many as 1,000 cases per 100,000 population. Nonepidemic periods have lower rates of occurrence, with approximately 5-10 cases for every 100,000 inhabitants in the region. The outbreaks in the African meningitis belt are usually a result of the serotype A group of N. meningitidis, although minor cases are at times due to serogroups C, X, and W-135. Individuals travelling to areas located within sub-Saharan Africa are at significant risk of contracting disease after extended contact with local inhabitants during the course of an epidemic (CDC)
Outbreaks of meningococcal disease are predominately found in the meningitis belt, but they also appear in the Unitd States. 90% of meningococcal diseases in the United States are a result of serogroups B and C. The average annual rate of invasive diseases caused by N. meningitidis is estimated to be 1.1 cases per 100,000 people or 2,600 cases annually. (PHC mening) Those most at risk for meningococcal disease are toddlers, with rates of incidence estimated to be 46% in children younger than 2 years. Approximately 25% of infections occur in patients older than 30 years. (PHC)
Morbidity and Mortality
The mortality rates of illness caused by N. meningitidis differs depending on the region, country, and age group (CDC manual). Typically, 5% to 10% of patients with meningococcal meningitis die, usually within a period of 24 to 48 hours of initial symptom presentation, despite prompt diagnosis and antibiotic therapy. Without treatment, individuals afflicted with meningitis have a 50% mortality rate. Among the patients that recover from invasive meningococcal diseases, approximately 10% to 20% have permanent sequelae including hearing loss, neurologic damage, or learning disabilities. (WHO) Other complications that occur infrequently include blindness, seizures, myocarditis, pericarditis, ataxia, pneumonia, conjunctivitis, and chronic meningococcemia. (PHC mening)
Virulence Factors
N. meningitidis utilize a variety of virulence factors to aid in its survival and proliferation in the host reservoir.
Capsules: The absence or presence of encapsulated N. meningitidis is highly dependent on the origin of sample. When isolated from carriers, the bacteria may be either capsulate or acapsulate, while samples isolated from cerebrospinal fluid or the bloodstream are consistently capsulate. The presence of capsules in pathogenic N. meningitidisserve to provide resistance against antibody, complement-mediated, or phagocytic destruction by the host immune response. Serogroups B, C, W-135, and Y incorporate sialic acids into the capsule, allowing for evasion by the immune response as sialic acids are also commonly found on several host cell surfaces. A phenomenon identified as capsule switching occurs between the aforementioned serogroups, a process which allows for horizontal exchange of the capsule operon, thereby resulting in anti-capsular antibodies to prove ineffective in eliminating the pathogen. (cs118)
LPS: Endotoxin is a cardinal virulence factor in N. meningitidis, inducing septic shock in patients by triggering pro-inflammatory mediator production. However, the LPS in this species are referred to as lipooligosaccharides (LOS) due to the lack of repeating O-antigens in the polysaccharide structure. Is it the oligosaccharides that contribute to the 12 differing immunotypes of N. meningitidis which are also subject to phase variation via frameshift mutations, allowing the pathogen to circumvent the host immune response. (carriage state)
Adhesion: Several structural and molecular features allow bacteria to adhere to the mucosal surfaces for colonization. N. meningitidis use filaments known as type IV pili located on its surface to adhere to the CD46 membrane coreceptors of host cells. (carriage state) Pili of the Neisseria genus are approximately 6 nm in diameter. In addition to pili, N. meningitidis also express Opa and Opc, two types of proteins found on the outer membrane of the bacteria. Through genetic discrepancy, Opa proteins frequently undergo antigenic variation. Host cells such as epithelial and endothelial cells express a family of receptors known as CEACAM (carcinioembryonic antigen-related cell-adhesion molecule) that bind to Opa proteins during adhesion, while Opc proteins bind to other specific receptors on host endothelial cells. Both types of proteins mediate adhesion and invasion of host cells to facilitate the spread of the pathogen. (cs118) In addition to Opa and Opc proteins, N. meningitidis strains also express and secrete many other proteins that bind to human cells. Amongst these proteins are lactoferrin- and transferrin-binding proteins that also assist the bacteria in binding iron, a fundamental growth factor for colonization and the spread of disease. (cs118)
