Nipah Virus: Difference between revisions
Mcquistons (talk | contribs) |
Mcquistons (talk | contribs) |
||
| Line 51: | Line 51: | ||
==REPRODUCTION OF PARAMYXOVIRUSES== | ==REPRODUCTION OF PARAMYXOVIRUSES== | ||
<br>As previously discussed, it is hypothesized there are many models of transmission of Nipah virus from species to species. It can also be assumed, because NiV is a paramyxovirus it acts very similar to other viruses within the family, the reproduction mechanism is very similar to influenza. The virion binds and fuses to the surface of a host cell via the F and G proteins. The lipid bi-layers are then melted and the viral nucleocapsid is released into the host cell. The negative sense viral RNA is transcribed to mRNA which acts as a template for more negative sense viral RNA. The viral RNA is used to make the necessary proteins (N,P,M,F,G,L,C,V,W) which congregate near the cell membrane. Once all the necessary proteins are assembled a new viral cell will bud off and infect other host cells. The new viral cells are able to fuse together and create a huge multinucleated cell called syncytia. A major different between the reproduction of paramyxoviruses and influenza is paramyxovirues are strictly reproduced in the cytoplasm. <br> | <br>As previously discussed, it is hypothesized there are many models of transmission of Nipah virus from species to species. It can also be assumed, because NiV is a paramyxovirus it acts very similar to other viruses within the family, the reproduction mechanism is very similar to influenza. The virion binds and fuses to the surface of a host cell via the F and G proteins. The lipid bi-layers are then melted and the viral nucleocapsid is released into the host cell. The negative sense viral RNA is transcribed to mRNA which acts as a template for more negative sense viral RNA. The viral RNA is used to make the necessary proteins (N,P,M,F,G,L,C,V,W) which congregate near the cell membrane. Once all the necessary proteins are assembled a new viral cell will bud off and infect other host cells [''FIGURE 6'']. The new viral cells are able to fuse together and create a huge multinucleated cell called syncytia [''FIGURE 6'']. A major different between the reproduction of paramyxoviruses and influenza is paramyxovirues are strictly reproduced in the cytoplasm. <br> | ||
==PATHOLOGY== | ==PATHOLOGY== | ||
Revision as of 18:34, 2 May 2013
INTRODUCTION
By Sam McQuiston
Throughout the history of the world humans have been plagued by diseases of various types and origins. Zoonotic diseases, or diseases which have the capability to jump species, animals to humans or vice versa, have been particularly troublesome and deadly. Zoonotic diseases are unique in that they are mainly caused by pathogens such as fungi, bacteria, parasites, or viruses. These pathogens typically survive in a reservoir host, which have immunity to the pathogen. The list of possible reservoir hosts capable of transmitting disease to humans is expansive; however the most common are apes, insects, rodents, and bats. The diseases are then passed to humans who come in contact with an infected animal through bites or scratches, an infected animal’s environment, or animal secretions such as saliva, feces, or mucus. Often these diseases have a higher virulence because of the lack of any immunity within the human population and the ease of transmission. Some more infamous zoonotic diseases are West Nile, Rabies, Ebola, and Dengue fever.
As of recent, more and more zoonotic diseases are emerging because of an increase in human and wildlife interaction. An increase in farming and or deforestation has resulted in humans and wildlife into the same habitat. A prime example of this is the emergence of the Nipah virus (NiV). NiV is a member of the Henipavirus genus in the Paramyxoviridae, and has become a growing concern of Southeast Asia and Australia.
Nipah virus is also a growing concern for the United States. The Center of Disease Control (CDC) has declared it a biosafety level 4 agent 6. This the highest biosafety level category, home to agents which can be distributed via aerosol transmission and have no treatment or vaccine. Similar biosafety level 4 agents are Ebola, Smallpox, and several hemorrhagic diseases. The CDC has also tagged the Nipah virus a Category C bioagent, the third highest priority agent category in regards to biological warfare 6. The availability, simplicity to produce and disperse, and high mortality rate of the Nipah virus make it possible for it to be used as a weapon of biological warfare.
FUN FACT

The Nipah virus was the virus which inspired the MEV-1 virus in the 2011 Hollywood hit “Contagion” [FIGURE 1].
MORPHOLOGY OF NiV
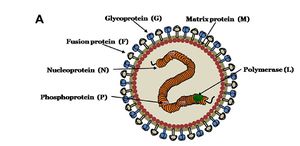
Nipah virus is in the newly created Henipavirus genus with the closely related Hendra virus and Cedar virus. The Henipavirus family is pleomorphic, meaning their shape is varied, and traditionally 40 to 600 nm in diameter [9]. The core of a virion contains a linear ribonucleprotein (RNP) comprising of negative sense single stranded RNA [FIGURE 2]. Also present in the RNP are three critically important proteins [FIGURE 2]. Nucelocapsid proteins (N) are tighly bound to the various nucleotides of the RNA strand [FIGURE 2]. N protein is the most abundant protein present and necessary for capsid structure. Phosphoproteins (P) and large polymerase proteins (L) are also bound to the RNA and aid RNA polymerase in transcribing RNA to mRNA to antigenomic RNA [FIGURE 2]. The virion is enveloped by a traditional lipid bilayer but “spiked” with fusion (F) and receptor-binding glycoproteins (G) [FIGURE 2]. The fusion proteins are responsible for fusing the viral membrane to the host membrane triggering the release of the contents of the virion. The receptor-binding glycoporteins are extremely specific and bind only to Ephrin B2 (EFNB2) surface proteins9. Specifically, NiV has been found to alternatively bind to EFB3 as well. The EFNB2 surface proteins are highly conserved across the mammalian lineage 9. On the underside of the lipid bilayer matrix proteins (M) are present for structural support and regulating the budding process. Other proteins, C, V, and W, are also present in the cytoplasm and involved in regulation of transcription and replication [FIGURE 2].
In regards to the Nipah virus genome, the exact structure is not completely understood. However because of the strong homology between Hendra virus and Nipah virus, a nearly identical structure is hypothesized. The negative sense single stranded RNA is of traditional 3’ to 5’ orientation. All the previously mentioned proteins are encoded by the RNA in the order of 3’-N-P-M-F-G-L- 5’12. Similar to all paramyxoviruses NiV RNA replication occurs in the cyptoplasm. All but the P gene are monocystronic, in that they code for a single protein. The P gene also encoded for the C, V, and W proteins which play a role in the virulence of NiV.
Interferons are released by host cells when under attack by a pathogen which enables intercellular communication. The intercellular communication is necessary for the triggering of immune cells which get rid of the pathogen. C, V, and W proteins, encoded by the P gene, have anti-interferon activity in that they block the transcription of interferon signaling. The process by which C, V, and W proteins block the signaling is still unknown.
NiV OUTBREAK HISTORY
The initial outbreak of encephalitis, later discovered to be a result of NiV, and first epicenter occurred in September of 1998 in several pig farms in a small town called Ipoh in northern Malaysia 13. NiV is thought to have been transmitted through southern Malaysia by the transference of infected pigs to a second epicenter located around Kampung Sungai Nipah 13. By more transport of infected pigs, as well as human-human transmission, NiV quickly spread into Singapore. This outbreak resulted in 276 infected patients with 106 deaths, a fatality rate of 38% 13. The main transmission of this outbreak is thought to be from the reservoir host to an intermediate host, to humans. This outbreak also resulted in a huge death rate of the pig population in Malaysia and Singapore.
Since the outbreak in 1998, a dozen outbreaks have since occurred in Bangladesh and India starting in 2001 13. These outbreaks have resulted in more respiratory disease and a fatality rate of up to 92% which have lead scientists to suspect a different strand of Nipah virus as the culprit 13. The transmission is thought to be directly from the reservoir host to humans, along with a higher human to human transmission rate.
The most recent outbreak occurred in early February of 2013, resulting in 12 infections and 10 deaths. Overall in Bangladesh, there have now been 188 infections with 146 deaths with a fatality rate of 77% 4.
OVERVIEW OF NiV TRANSMISSION

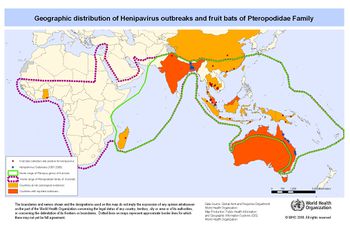
As previously stated, zoonotic diseases are spread from animals to humans via a reservoir host. However there can also be an intermediate host, in which the virus amplifies itself. However this intermediate host is not immune to the disease. Through analysis of urine and saliva collected from a suspected reservoir host from the original outbreak in Malaysia, the reservoir host has been identified as the Pteropus fruit bat [FIGURE 4].
NiV infection has been found in 5 of 14 fruit bat species, with the highest being Pteropus hypomelanus with a 31% infection rate 9. Despite having a relatively high infection rate, the species of Pteropus fruit bats are not susceptible to the virus. The virus is primarily transmitted via body secretions or partially eaten fruit.
Pigs have been found to be particularly susceptible to NiV as well as highly contagious to each other. Pigs have been identified as an intermediate and an amplifying host. An amplifying host is defined as a host in which the pathogen can become so prevalent a vector, such as a mosquito, can become infectious. Pigs transmit NiV differently than bats in that they shed the virus primarily by coughing and the expulsion of respiratory secretions and saliva. NiV has not been isolated in pig urine. Pigs are primarily infected directly from Pteropus fruit bats or other pigs.
Humans have been found to be infected by three different pathways, Pteropus fruit bat to human, pig to human, and or human to human. Most recent transmission of NiV has been a result of human to human transmission through close contact through respiratory secretions or urine.
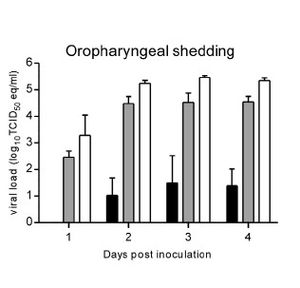
Although not completely understood, the molecular mechanism of Nipah virus transmission is hypothesized to be by fomites. Fomites are non-living objects which are able to carry infectious material like vectors. Currently this is the main hypothesis because of the increased number of NiV infections being picked up in hospitals. It is unknown how long the virus can survive outside a host. Because of the heavy prevalence of virus transmission via respiratory secretions it is assumed the main site of replication occurs in the tonsils of an infected host 6. This is supported by research done in the transmission of NiV in hamsters. de Wit et al (2011) observed viral shedding of hamsters inoculated with three different doses of varied strength of NiV [FIGURE 3]. The researchers then examined varied forms of shedding of the virus nasally, oropharyngeally, urogenitally, and rectally. The results show, regardless of the dosing, all three dosages resulted in positive viral loads in the oropharyngeal region as soon as two days post inoculation 3 [FIGURE 3].
The fact that the reservoir and intermediate hosts are Pteropus fruit bats and pigs, respectively, is a primary concern for researchers. Pteropus fruit bats are a migratory species capable of flying long distances. Pigs are also a primary export of Southeast Asia as well as a popular farm animal in the same region. The combination of these two key aspects of the Pteropus fruit bat and pigs makes the transmission of the Nipah virus to other parts of the world extremely easy.
NiV infections have also been identified in cats, dogs, and horses 9.
MALAYSIA/SINGAPORE OUTBREAK TRANSMISSION OF NiV
It is believed the transmission from the Pteropus fruit bats to an intermediate host is due to a growing overlap in habitats between the two species [FIGURE 5]. Malaysia is home to a large number of both pig farms and fruit orchards, often located relatively close to each other. The close proximately is thought to have allowed Pteropus fruit bat urine, feces, and or infected fruit to drop onto the pigs or into their habitat. It was speculated pigs had become an amplifying intermediate host because of the enormous death toll in the pig population as well as a majority of the humans infected had a large amount of contact with pigs. In the years after the outbreak NiV antibodies were identified in pig populations in Malaysia. As stated above, a large majority of humans infected had direct contact with pigs. The virus was also able to spread from farm to farm through transportation of unidentified infected pigs. Most likely the workers whom were infected can in direct contact with saliva or other respiratory secretions from an infected pig.13
BANGLADESH/INDIA OUTBREAK TRANSMISSION OF NiV
Because of the variation in the effect and fatality rate of the Nipah virus outbreak in Bangladesh and India [FIGURE 5], a slightly altered transmission path is suggested. It is suggested a more direct path was taken from the reservoir host, Pteropus fruit bats, directly to humans. The large consumption of date palm sap by humans is looked to be where the virus was picked up. Pteropus fruit bats feed heavily on date palm trees, licking the sap which contaminated the sap. Improper cooking of the sap resulted in humans ingesting the infected sap. A second mode of transmission is through contact with infected Ptreopus fruit bat feces, urine, or saliva.
Although human to human transmission had not been suggested in the Malaysia/Singapore outbreak, it is believed to have been extremely prevalent. It is believed nosocomial transmission, passing of a pathogen in a hospital setting due to improper hygienic/sterilization methods, was the causation in up to 75% of cases 10. Data collected from various hospitals in Bangladesh and India where patients infected with NiV were treated showed a high amount of NiV RNA on hospital surfaces.
REPRODUCTION OF PARAMYXOVIRUSES
As previously discussed, it is hypothesized there are many models of transmission of Nipah virus from species to species. It can also be assumed, because NiV is a paramyxovirus it acts very similar to other viruses within the family, the reproduction mechanism is very similar to influenza. The virion binds and fuses to the surface of a host cell via the F and G proteins. The lipid bi-layers are then melted and the viral nucleocapsid is released into the host cell. The negative sense viral RNA is transcribed to mRNA which acts as a template for more negative sense viral RNA. The viral RNA is used to make the necessary proteins (N,P,M,F,G,L,C,V,W) which congregate near the cell membrane. Once all the necessary proteins are assembled a new viral cell will bud off and infect other host cells [FIGURE 6]. The new viral cells are able to fuse together and create a huge multinucleated cell called syncytia [FIGURE 6]. A major different between the reproduction of paramyxoviruses and influenza is paramyxovirues are strictly reproduced in the cytoplasm.
PATHOLOGY
Broadly, the Nipah virus causes a multitude of problems within the infected host. However NiV presents itself in a varied set of symptoms depending on the infected species. In general the main mode of dissemination with each host, regardless of species, is by inducing syncytial cell formation [FIGURE 6]. These large multinucleated cells then spread rapidly through the vascular tissue of the infected host.
Incubation time in pigs varies but is usually short between 2 and 10 days. The Nipah virus primarily attacks the respiratory system, which is supported by the finding of high concentrations of viral antigens are found in the respiratory tract and lung epithelium 2. Pigs infected with NiV have shown to have an acute fever with symptoms of a respiratory infection which produces a “barking cough”. This “barking cough” has been identified as a telltale sign of Niv infection in pigs. Niv infection also produces signs of nervousness, twitching and trembling, hemorrhaging, and lesions in both the brain and lungs. Although very virulent and contagious among pigs, the mortality rate is very low at roughly 1-5% 2.
Unlike Nipah virus infection in pigs, human infection is particularly encephalitic. The onset of a fever after and incubation period as short as two days or as long as a month is the first sign of infection. Common signs of a viral infection such as headache and drowsiness occurred along with the fever. Doctors are able to distinguish a Nipah viral infection by the distinctive symptoms of encephalitis, vasculities, neurological deficits due to necrosis, thrombosis, and ischemia 13. The cerebrospinal fluid is also heavily impacted with an increase of proteins and dead cells present. The heavy encephalitic nature of NiV infection in humans also results in brain lesions, often times causing a relapse of encephalitis in patients who had recovered from NiV infection. Humans infected with NiV have a much higher fatality rate ranging from 40% to 75% depending on the location of the infection 12.
DETECTION AND TREATMENT OF NiV
There has yet to be a standard protocol in detecting Nipah viral infections but the most common process used currently is virus isolation from tissue samples. In all species NiV can be detected and isolated from the kidneys, cerebrospinal fluid, and the liver. Polymerase chain reaction, enzyme-linked immunosorbent, and immunofluorescence assays are also viable detection strategies 14.
Currently there are no vaccines or drugs which can cure or treat a NiV infection. The primary approach is to treat the symptoms as best as possible in hope to control the infection. Creating a vaccine is currently extremely difficult because the mutation rate of RNA viruses is extremely high as is most zoonotic diseases, of which Nipah virus is both 9. However several studies have been conducted in targeting the F and G proteins which would inhibit binding of the NiV cells to any host cell 9.
CONCLUSION: THE FUTURE OF NiV
Several steps have been taken in the right direction towards eradicating this extremely harmful disease. After the initial outbreak of Nipah virus in 1998, the Malaysian and Singaporean governments developed a two phase plan in hope to control any future outbreak. Phase one was set to eliminate a majority of the pigs present within the country. Phase two introduced an antibody testing protocol to regulate and observe farms which may be of high risk for an outbreak 7. Both countries also banned the transportation of pigs within the respective countries as well as initiated an educational program to aid the farms in proper handling and the virus itself. As previously stated some initial ground work has also been laid in identifying a possible vaccine which inhibits the activity of F and G proteins on the viral cell 9.
Despite these positive steps, Nipah virus should be at the top of the list of major concerns for the human race for several reasons:
1.) Increased human to human transmission in recent outbreaks: Transmission of NiV has now become a concern in many hospitals in Southeast Asia. There have been several cases of doctors becoming infected from treating patients as well as infections resulting from contact with corpses. The more recent outbreaks in Bangladesh and India are suggested to have an estimated 75% or more of the known infections resulting from humans to humans transmission. The main mode of transmission from human to human is hypothesized to be through respiratory secretions and close contact. This mutated strain of NiV has the potential to be extremely detrimental in densely populated cities.
2.) Rising fatality rate in humans: The most recent outbreak in Bangladesh in February of 2013 resulted in increasing the overall fatality rate of Nipah virus infection in Bangladesh to 77% 4.
3.) Lack of knowledge of molecular mechanisms of infection: The molecular mechanisms of how the virus is passed from species to species are still fairly unknown.
4.) Shared habitats: With a rapidly growing human population in the world, particularly in Southeast Asia, there is an increase of overlapping of habitats between humans, pteropus fruit bats, and pigs. This only increases the chance of transmission of NiV between the species as well as the risk of more outbreaks.
5.) Pteropus fruit bat migration and pig dependence: Pteropus fruit bats are migratory animals which can survive in a wide range of environment, while much of rural Southeast Asia is dependent on their pig farms as a source of income and food. The combination of these two aspects opens up many pathways to many new countries and new populations of humans with no previous exposure to NiV.
6.) NiV is an RNA virus and a zoonotic virus: RNA viruses have a high mutation rate which enables them to keep a leg up on both vaccines and host immune systems. Zoonotic viruses also have a high mutation rate. Because NiV is both of these, it is hypothesized it has an extremely high rate of mutation.
References
[1] Bossart, K.N., Tachedjian, M., McEachern, J.A., Crameri, G., Zhu, Z., Dimitrov, D.S., Broder, C.C., Wang, L.F., 2008. Functional studies of host-specific ephrin-B ligands as Henipavirus receptors. Virology 372, 357–371.
[2] Cobey, S. "Nipah Virus." HERG. The Henipavirus Ecology Collaborative Research Group, 2005. Web. 19 Apr. 2013.
[3] de Wit E, Bushmaker T, Scott D, Feldmann H, Munster VJ (2011) Nipah Virus Transmission in a Hamster Model. PLoS Negl Trop Dis 5(2012): e1432.doi:10.1371/journal.pntd.0001432
[4] "Fresh Outbreak of Nipah Virus in Bangladesh." Editorial. IRIN 5 Feb. 2013: n. pag. Www.irinnews.org. IRIN. Web.
[5] Gurley, E.S., Montgomery, J.M., Hossain, M.J., Islam, M.R., Molla, M.A., Shamsuzzaman, S.M., Akram, K., Zaman, K., Asgari, N., Comer, J.A., Azad, A.K., Rollin, P.E., Ksiazek, T.G., Breiman, R.F., 2007b. Risk of nosocomial transmission of Nipah virus in a Bangladesh hospital. Infect. Control Hosp. Epidemiol. 28, 740–742.
[6] "Nipah Virus." Www.CIDRAP.umn.edu. University of Minnesota, n.d. Web. 19 Apr. 2013
[7] Nor MNM. Emergency report on Nipah to the OIE. Disease Information 1999 May 28;12(20)
[8] "Paramyxoviridae." Www.MicrobeWiki.kenyon.edu. MicrobeWiki, 8 Aug. 2010. Web. 19 Apr. 2013.
[9] Rockx, Barry, Richard Winegar, and Alexander N. Freiberg. "Recent Progress in Henipavirus Research: Molecular Biology, Genetic Diversity, Animal Models." Antiviral Research 95 (2012): 135-49. Print.
[10] Sazzad, Hossain M.S., M. Jahangir Hossain, Emily S. Gurley, Kazi M.H. Ameen, Shahana Parveen, M. Saiful Islam, I. Faruque, Goutam Podder, Sultana S. Banu, Michael K. Lo, Pierre E. Rollin, Paul A. Rota, Peter Daszak, Mahmurdur Rahman, and Stephen P. Luby. "Nipah Virus Infection Outbreak with Nosocomial and Corpse-to-Human Transmission, Bangladesh." Emerging Infectious Diseases 19.2 (2013): 210-17. Www.cdc.gov. Center of Disease Control, Feb. 2013. Web.
[11] St. Georgiev, Vassil, PhD. "Impact on Global Health." National Institute of Allergy and Infectious Diseases 2 (2007): 143-150
[12] Thong W.K, W.J. Shieh, K. Shalini, N. Karim, W.Abdullah, J Guarner, C. S. Goldsmith, K.B. Chua, S.K. Lam, C.T. Tan, K. J. Goh, H.T. Chong, R. Jusoh, P.E. Rollin, T.G. Ksiazek, S.R. Zaki, The Nipah Virus Pathology Working Group, Nipah Virus Infection: Pathology and Pathogenesis of an Emerging Paramyxoviral Zoonosis, The American Journal of Pathology, Volume 161, Issue 6, December 2002, Pages 2153-2167, ISSN 0002-9440, 10.1016/S0002-9440(10)64493-8.
[13] Thong, Wong Kum. "PATHOLOGY AND PATHOGENESIS OF NIPAH VIRUS INFECTION IN HUMANS AND ANIMAL MODEL." Diss. UNIVERSITY OF MALAYA, 2008. Faculty of Medicine, Dec. 2008. Web.
[14] WHO. Nipah virus fact sheet. Sep 2001
Edited by Sam McQuiston, student of Joan Slonczewski for BIOL 238 Microbiology, 2013, Kenyon College.
