Ocular Infection by Chlamydia trachomatis: Public Health Responses to Pathology
Introduction and Significance
By Lydia Wolf
The World Health Organization estimates ocular infection by the organism Chlamydia trachomatis to affect approximately 1.8 million people worldwide[1]. Repeated infection by the intracellular parasite, which typically begins during childhood, causes inflammation of the skin epithelium lining the inside of the eyelids called the conjunctiva. The chronic infection and inflammation of the conjunctiva causes the eyelashes of the upper lid to turn in and rub directly against the surface of the cornea. This rubbing against the cornea leads to scarring and, when left untreated by a trachiasis surgical procedure, ultimately leads to blindness…making it the leading cause of infectious blindness worldwide [2].
The pathogenic organism Chlamydia trachomatis is transmitted through direct personal contact of discharge from the mucous membranes of the eyes and nose, as well as flies that have been in contact with infected membranes, causing trachoma. The bacteria can also be transmitted to a newborn during delivery from a mother with a genital chlamydial infection causing neonatal conjunctivitis [3]. Ocular trachoma is especially prevalent in poor and rural developing regions affected by environmental risk factors which include crowded living conditions, and poor water sanitation facilities, as well as limited access to health care. Children and women living in these regions are a population of particular concern, as the family unit is a significant vehicle for transmission by direct contact with previously infected family members.
During the early 19th Century, trachoma (although already present in Europe before this time) became prevalent throughout Europe due to close contact between European and Egyptian soldiers during the Napoleonic Wars. Trachoma became an increasing public health concern in Europe following the Napoleonic Wars, and was a concern for the U.S. government regarding European immigration during the early 20th century who maintained strict trachoma inspections before the embarkation of immigrants in Europe. The discovery of trachoma’s causative agent did not occur until around 1907 when the two Austrian researchers, Halberstaedter and Prowazek, discovered the cytoplasmic inclusion bodies in scrapings from infected patients by staining and light microscopy, which were then used to inoculate the eyes of orangutans showing how the trachoma infection was spread between individuals. Further research following this initial discovery began to link ocular trachoma to newborn conjunctivitis and sexually transmitted infections in the female genital epithelium [4].
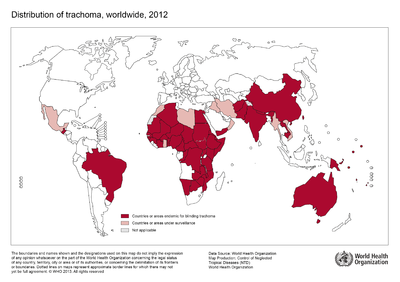
Although no longer a major health concern in Europe or the United States, trachoma remains prevalent in certain endemic regions of the developing world. The World Health Organization estimates the disease to be endemic in approximately 50 nations worldwide across Africa, Asia, and regions of the Middle East with approximately 232 million people living in these areas at risk for infection [5]. Public health campaigns are being conducted across these regions to meet goals outlined twenty years ago at the 1996 launching of the “WHO Alliance for the Global Elimination of Trachoma by the year 2020” [5]. The public health response campaigns working towards control and prevention of ocular trachoma, typically implement the WHO recommended “SAFE” strategy consisting of both environmental and medical interventions [2].
Classification, Structure, and Developmental Cycle of Chlamydia trachomatis
Chlamydia trachomatis is a gram-negative obligate intracellular pathogen of the Chlamydiacae family. Originally thought to be a virus, because of the organism’s dependence on key metabolic intermediates (including ATP) from biosynthetic pathways of the eukaryotic host, C. trachomatis is now known to have a cell wall, DNA, RNA, and ribosomes and is classified as Bacteria [6]. The group of small non-motile, cocci-shaped bacteria consists of a single genus, Chlamydia, and includes three species.
Chlamydia psitacci is a known pathogen causing infection in birds and a number of mammalian species, including humans. The other two Chlamydial species, C. trachomatis and C. pneumoniae, are largely limited to causing infection in human hosts including ocular trachoma infections and genital-tract infections by C. trachomatis, and infection of the respiratory system by C. pneumoniae. Multiple distinct variations called Serotypes exist within the species-level classification of C. trachomatis based on differences in cell wall proteins, with each displaying different selectivities for certain host tissue types [7].
Serovars of C. trachomatis are defined by letters A-K as well as Ba, Da, Ia, and Ja. Among these, serovars A, B, Ba, and C are know to cause trachoma while serotypes D-K typically cause infections of the genital-tract[3]. The unique clinical presentation and diagnosis of an ocular disease resulting from infection by C. trachomatis depends on the serovar infecting the host tissues. A serovar which typically causes infection of the genital-tract may additionally infect the mucosal tissues of an infant’s eyes during passage through the mother’s infected birth canal, causing a neonatal conjunctivitis similar to the clinical presentation of trachoma.
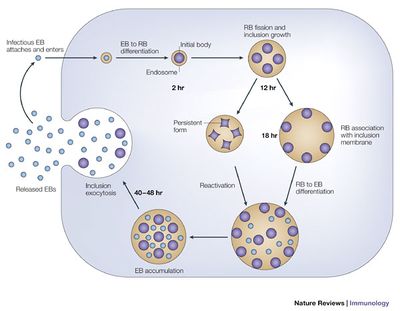
Unable to grow or replicate outside of a eukaryotic host cell, Chlamydia have developed a unique parasitic developmental cycle shown in Figure 2, in which the organism alters between the metabolically inert elementary body (EB) and the reticulate body (RB) capable of multiplying by binary fission within a host cell [8]. In the initial stage of infection of the host cell, the spore-like metabolically inert elementary body, attaches and enters the host cell via bacterial adhesin and receptor proteins of the host cell (although the nature of the adhesin and receptor molecules involved are not yet known). The elementary body is next taken up into an intracellular membrane-bound vacuole called an inclusion.
During the primary differentiation stage of the cycle, elementary bodies differentiate into the larger, metabolically active reticulate bodies (BD), which proceed to undergo cycles of binary fission in the middle stages of the cycle. Transcription of bacterial protein genes begins during this differentiation step with studies suggesting genes expressed during this stage are involved with nutrient acquisition as well as modification of the inclusion to prevent lysosomal fusion in which the pathogenic bacteria can be killed by the host’s immune response production of phagolysosomes. Following cell division, the elementary bodies redifferentiate into elementary bodies during a period of secondary differentiation. Genes expressed during this stage in the cycle include genes that encode outer-membrane proteins, and proteins involved in chromosome condensation. These elementary bodies are then released outside the cell to infect neighboring host cells upon lysis of the host epithelial cell [8].
Under conditions of stress, as imposed by host immune suppression, nutrient deprivation, and antibiotic exposure, Chlamydia species are known to undergo an altered growth cycle in which the bacteria survive in a culture-negative state during unfavorable growth conditions [9]. Several studies have indicated evidence of reactivation of persistent chlamydial growth and infection in vivo. One such report examined the recurrence of ocular trachoma infection in adults who had been diagnosed with the infection while living in an endemic area during childhood, who developed active trachoma years later as adults while living in a non-endemic area, indicating infection due to persistent infection reactivation rather than a re-infection [9]. The complicated biphasic developmental life cycle of C. trachomatis, as well as it’s ability to survive in a persistent non-culturable growth phase and reoccur in the host years later, has significant implications for consideration of public health treatment strategies for ocular trachoma with regards to mass antibiotic administration.
Pathology and Immune Response of Chlamydia trachomatis
Trachoma was initially believed to be caused by a single infection from C. trachomatis, however recent research has revealed the importance of repeated episodes of infection for the development of chronic inflammation and scarring of the conjunctiva characteristic of the disease. Various studies of ocular and genital tract infections from C. trachomatis in human and animal models have shown the tissue damage and scarring characteristic of the disease pathology to be caused by cell mediated immune responses against bacterial antigens
[10]. Appearance of lymphoid follicles, collections of human immune lymphoid cells that include B and T lymphocytes, on the inner eyelid skin layer called the conjunctiva characterize active infection. The follicles typically appear as small yellow to white bumps, and are important clinical signs for the clinical diagnosis of active trachoma infection by C. trachomatis. Between the follicles that develop, plasma cells, macrophages, granular leukocytes, and B and T cells characterize the infected conjunctiva. After years of chronic infection and inflammation, the normally loose, vascular stroma of the conjunctiva is replaced by thick scar tissue [3].
The host immune response to infection by C. trachomatis has a role in the resolution of active infection. A study examining the relationship between age and incidence and duration of active trachoma found a threefold reduction in incidence of the disease with age of a given cohort. The age-dependent resolution of the disease, with older subjects having a shorter duration of untreated infection, suggests that acquired immunity of the host plays a role in clearing the infection [11]. The same cellular immune mechanisms, which appear to play a role in infection resolution, also contribute to the pathogenesis of the disease through inflammation and scarring.
The human immune response to the bacterial infection of Chlamydia trachomatis provides incomplete protection from re-infection, which is partially due to the many immune evasion strategies of the infective organism. Chlamydia trachomatis is able to avoid detection by host antibodies by having an antigenically diverse set of surface proteins. The unique developmental cycle of the organism shown in figure 2 also contributes to the evasion of immune responses produced by the host cells. The ability of the bacteria to persist in an alternative non-replicating form (similar to an endospore) allows resistance during conditions of nutrient deprivation and antibiotic exposure. In addition, replication and growth within an inclusion inside the host cell limits exposure to host antibodies. These evasion mechanisms might explain why the infection takes so long to clear despite the host’s innate immune response to infection and treatment with antibiotics [12].
Clinical Presentation and Diagnosis of Ocular Trachoma
Active trachoma is most prevalent in children living in geographic areas endemic for the disease. The disease can be divided into two clinical manifestations, the first of which are defined as the acute “active” stages of the disease which occur most frequently in pediatric patients with little to no symptom complaint, but may include symptoms of pain and light sensitivity as well as mucous-like discharge from red or irritated eyes [7]. The active stage of the disease is characterized by follicles (small yellow-white raised nodules on the surface of the conjunctiva), and papillae (elevations on the conjunctival tissue with a central vascular core), which are both macroscopically visible. The other clinical manifestation of ocular trachoma can be defined by the late-stage, chronic manifestation of the disease which results from repeated infection of active trachoma (with periods of resolution between infection episodes) over several years. In this manifestation, upper-lid conjunctiva scarring accumulates, causing the eyelashes to turn inward and rub against the surface of the cornea in a painful process called trachiasis.
Clinical examination of the eye, including inspection of the lashes and especially the upper conjunctiva and cornea, is the most prevalent method for diagnosing ocular trachoma – especially in the field where laboratory testing can be both complex and costly to implement [2]. Although follicles are not necessarily indicative of trachoma, they are a strong predictor of the disease in endemic areas and contribute to the clinical diagnosis of the active stage of the infection. Papillae, on the other hand, are a poor indicator of trachoma as they are an observed tissue response to inflammation caused by a wide range of bacterial, viral, and allergic inflammatory disorders.
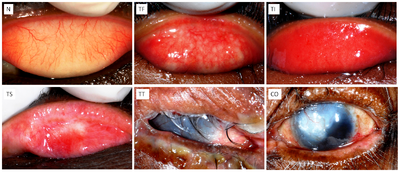
In an attempt to standardize the clinical diagnosis of ocular trachoma in the field, several grading systems have been developed over the years. Most now use the WHO simplified grading system which is based on five selected key signs to aid in the diagnostic process of both experienced medical professionals and non-specialists working in the public health field [7]. The WHO simplified grading system consists of five gradings based exclusively on changes observed in the upper conjunctiva as shown in Figure 3. Trachomatous inflammation, follicular (TF) indicates a moderately active form of the disease, when five or more follicles are observed on the surface of the upper conjunctiva. Trachomatous inflammation intense (TI) is a slightly more severe form of active trachoma in which there is a pronounced inflammation and thickening of the upper conjunctiva. Trachomatous scarring (TS) occurs when visible scarring is observed in the upper conjunctiva. Trachomatous trachiasis (TT) is diagnosed when an eyelash is rubbing directly against the cornea or there is evidence of recent removal of an in-turned eyelash. This presentation of the disease is the common stage for surgical intervention with a trachiasis procedure which aims to relieve discomfort, improve vision, and prevent progression to the next stage of the disease: corneal opacity (CO) in which there is visible corneal opacity over the pupil causing blindness.
In addition to the more common form of diagnosis by clinical examination of changes to the upper conjunctiva tissue, several laboratory assays aid in the clinical diagnosis of infection by Chlamydia trachomatis. Microscopy of stained tissues from conjunctival scrapings of the infected individual provides one method of diagnosis, in which the characteristic inclusions appear as darkly stained masses in the host’s epithelial cells. Other, more sensitive diagnostic tests include isolation in cell culture, direct fluorescent antibody testing, enzyme immunoassays which detect presence of chlamydial antigens present in a conjunctival swabbing, and nucleic amplification tests [7].
Public Health Initiatives Targeting Infectious Ocular Trachoma

The most commonly implemented public health response for prevention and containment of ocular infection by Chlamydia trachomatis in regions endemic for the disease is the SAFE strategy developed by the World Health Organization. The strategy sets specific goals for clinical treatment of individual cases of active trachoma including: Surgery to treat trachiasis, and Antibiotic administration, as well as community-level public health prevention strategies which include Facial cleanliness, and Environmental changes [13] .
Mass antibiotic administration for community-level control of trachoma remains an integral component to the SAFE program, with recent research conducted in the field indicating the need for repeated distribution programs of oral azithromycin to ensure elimination of infection within a community . [14] While a single mass distribution of antibiotics has been shown to greatly reduce the occurrence of ocular chlamydial infection within a given community, clinical signs of active infection have been observed to reoccur within the population several months after the initial treatment [15]. Simulations using mathematical models for epidemiological transmission of trachoma testing the effects of several different treatment programs have suggested elimination of infection in larger areas may require repeated biannual antibiotic administration [16].
While treatment of active infection with azithromycin repeatedly yields positive results, the generic version of patented Zithromax manufactured by the US drug manufacturing company PFIZER, the availability of the antibiotic is limited in several endemic countries due to patent laws [2] .
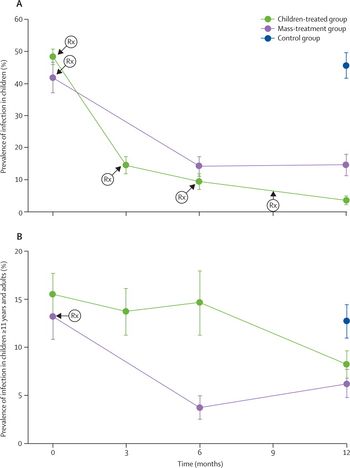
A recent study funded by the National Institute of Health examined the protective effect to untreated individuals from a mass antibiotic administration program which targeted treatment to a given cohort of the population, similar to the herd-immunity protection strategy often used in vaccination programs. In the study, 24 regional districts in Amhara, Ethiopia were randomized, with children aged 1-10 years in 12 districts receiving oral azithromycin treatment four times in a year, and delayed azithromycin treatment until after the study for the other 12 districts
[17]. The researchers then examined the prevalence of ocular infection in untreated children, 11 years and older, before the start of a quarterly antibiotic distribution and after 12 months in both the treated and control test groups (each composed of the 12 regional districts). The results of the study, shown in the figure at right, indicated decreased ocular infection in the untreated older children and adults living in the districts in which quarterly antibiotic distribution was targeted to the youngest population in the community. In addition to the form of herd protection which was observed, the researchers found that young children (who play a major role in the transmission of infective trachoma), receiving quarterly antibiotic treatments were less likely to be infected at 12 months compared to children from communities in which a single mass dose treatment strategy was implemented
[17].
Although not yet a clinical problem observed in the field where trachoma is endemic, there is some evidence within the literature that antibacterial-resistant strains of Chlamydia trachomatis could develop in regions with extensive antibiotic use over time
[18]. If evidence for resistance were observed, there could be a major effect on the strategies currently being implemented and programs might have to emphasize environmental prevention strategies to a greater degree. Identifying populations within a community contributing to high rates of prevalence and transmission of ocular chlamydial infection leading to trachoma has been important for implementing both cost-effective and successful treatment and prevention programs. Directing repeated antibiotic distribution to young children is both economically viable, due to the limited availability and high cost of the treatment, and a clinically successful method of control of the spread of infection through herd-immunity characteristic of vaccination programs.
Implications for a Future Vaccine
While a vaccine for ocular chlamydial infection by C. trachomatis would provide a significant advantage for trachoma control, its development has been unsuccessful to this point due to the challenging nature of both the pathology of the disease as well as the protective host immune responses during active infection and late-stage manifestations of the disease. Cell mediated immune responses, which release various mediators in the presence of the bacterial antigens causing the inflammation and scarring, play an important role in the candidates for a vaccine antigen. Genetic vaccination methods, which stimulate the host cellular immune response to produce protein antigens by injecting the host cell with genetically engineered DNA, pose a potential solution to the challenging nature of the vaccine development for C. trachomatis infections. A potential target for a CMI vaccine might be components of the type III secretion system discovered in C. trachomatis , which are known to contribute to the virulence of certain gram-negative bacteria [19].
References
- ↑ World Health Organization. Trachoma. May 2015. Web. 20 Apr. 2016
- ↑ 2.0 2.1 2.2 2.3 Kuper H, Solomon AW, Buchan J, Zondervan M, Foster A, et al. (2003) A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis. 3: 372–381
- ↑ 3.0 3.1 3.2 Mabey, D., Solomon, A., and Foster, A. (2003) Trachoma. The Lancet. 371(9628): 223-229.
- ↑ Schlosser, Katherine (2016). Trachoma Through History. International Coalition for Trachoma Control. Web. 20 Apr. 2016
- ↑ 5.0 5.1 “Trachoma”. World Health Organization. May 2015. Web. 20 Apr. 2016
- ↑ Becker, Y. Chlamydia. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 39
- ↑ 7.0 7.1 7.2 7.3 Solomon, Anthony W. et al. “Diagnosis and Assessment of Trachoma.” Clinical Microbiology Reviews 17.4 (2004): 982–1011. PMC. Web. 24 Apr. 2016
- ↑ 8.0 8.1 AbdelRahman, Y., and Belland, R. (2005). The chlamydial development cycle. FEMS Microbiology Reviews. (5): 949 – 959.
- ↑ 9.0 9.1 R., Mathews, S., Mukhopadhyay, S., et al. (2004). Chlamydial persistence: beyond the biphasic paradigm. Infection and Immunity 72(4): 1843-1855.
- ↑ Hu VH, Holland MJ, Burton MJ (2013) Trachoma: Protective and Pathogenic Ocular Immune Responses to Chlamydia trachomatis. PLoS Negl Trop Dis 7(2): e2020.
- ↑ Bailey, R., Duong, T., Carpenter, R., et al. (1999) the duration of human ocular Chlamydia trachomatis is age dependent. Epidemiol. Infet (123): 479-486
- ↑ Brunham, Robert C., and Jose Rey-Ladino. “Immunology of Chlamydia Infection: Implications For a Chlamydia Trachomatis Vaccine.” Nature Reviews Immunology 5.2 (2005): 149-161. Academic Search Premier. Web. 23 Apr. 2016
- ↑ “Water-Related Diseases.” WHO. N.p., n.d. Web. 25 Apr. 2016
- ↑ Kathryn J et al. A Rationale for Continuing Mass Antibiotic Distributions for Trachoma. BMC Infectious Diseases 7 (2007): 91. PMC. Web. 25 Apr. 2016.
- ↑ Chidambaram, J.D., Alemayehu, W., Melese, T. et al. (2006) Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. JAMA, 295: 1142-1146
- ↑ Ray, Kathryn J et al. A Rationale for Continuing Mass Antibiotic Distributions for Trachoma. BMC Infectious Diseases 7 (2007): 91. PMC. Web. 25 Apr. 2016.
- ↑ 17.0 17.1 House, J., Ayele, B., Porco, T., et al. (2009) Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet, 373: 1142-1118
- ↑ Binet, R., and Maurelli, A., (2005) Frequency of spontaneous mutations that confer antibiotic resistance in Chlamydia spp. Antimicrobial Agents and Chemotherapy. 49,7: 2865–2873.
- ↑ Beagley, K., and Timms, P. (2000). Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development. Journal of Reproductive Immunology 48(1): 47-68
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
