Phage Mediated Biocontrol of Food Borne Bacteria: Difference between revisions
Tina gao246 (talk | contribs) No edit summary |
Tina gao246 (talk | contribs) No edit summary |
||
| Line 65: | Line 65: | ||
===Bacterial Resistance to Phage Therapy=== | ===Bacterial Resistance to Phage Therapy=== | ||
Bacteria and their bacteriophage are constantly co-evolving. A study showed that E. coli O157, when incubated with phage PP01 for 200 hours, developed a series of mutants which differed in colony morphology, nature of phage receptors OmpC and LPS, and phage susceptibility [7]. The phage responded by evolving a broadened host range [7]. A trade off was observed between resistance to phage and competitiveness with parental strains for resources. For phage resistant strains to be selected for in the wild, they must also compete with many other strains that do not feel this phage pressure (unlike competing again only the phage-susceptible ancestor in the laboratory) [7]. If phage selective pressure is low, such mutants cannot be expected to present any danger in long-term phage based intervention [1]. Depending on the phage however, many bacteria are favoured in this co-evolutionary arms race (some resistance in certain strains even come without a metabolic cost) [7], thus bacterial resistance may still pose to be a problem in the future. | Bacteria and their bacteriophage are constantly co-evolving. A study showed that E. coli O157, when incubated with phage PP01 for 200 hours, developed a series of mutants which differed in colony morphology, nature of phage receptors OmpC and LPS, and phage susceptibility [7]. The phage responded by evolving a broadened host range [7]. A trade off was observed between resistance to phage and competitiveness with parental strains for resources. For phage resistant strains to be selected for in the wild, they must also compete with many other strains that do not feel this phage pressure (unlike competing again only the phage-susceptible ancestor in the laboratory) [7]. If phage selective pressure is low, such mutants cannot be expected to present any danger in long-term phage based intervention ([http://www.ncbi.nlm.nih.gov/pubmed/20214608 1]). Depending on the phage however, many bacteria are favoured in this co-evolutionary arms race (some resistance in certain strains even come without a metabolic cost) [7], thus bacterial resistance may still pose to be a problem in the future. | ||
===Commercial Production of Phages=== | ===Commercial Production of Phages=== | ||
In order for phages to be effective in phage-mediated biocontrol, studies must be tested under conditions which resemble commercial practices. For zoonotic bacteria such as Salmonella, there is need to determine the optimal timing and delivery of bacteriophage in a real-life poultry industry setting [3]. In order to have this intervention be scaled up for commercial production, cost-effectiveness vs. efficacy in real-life application will need to be assessed [1]. Market acceptance by the food industry and the consumer will need to occur before it can be considered an ideal antibacterial agent ([http://www.ncbi.nlm.nih.gov/pubmed/20214608 1]). | In order for phages to be effective in phage-mediated biocontrol, studies must be tested under conditions which resemble commercial practices. For zoonotic bacteria such as Salmonella, there is need to determine the optimal timing and delivery of bacteriophage in a real-life poultry industry setting [3]. In order to have this intervention be scaled up for commercial production, cost-effectiveness vs. efficacy in real-life application will need to be assessed ([http://www.ncbi.nlm.nih.gov/pubmed/20214608 1]). Market acceptance by the food industry and the consumer will need to occur before it can be considered an ideal antibacterial agent ([http://www.ncbi.nlm.nih.gov/pubmed/20214608 1]). | ||
==References== | ==References== | ||
Revision as of 05:18, 21 November 2012
Introduction
The [phyllosphere] refers to the above-ground surfaces of a plant as a habitat for microorganisms, with a heavy emphasis on leaf surfaces. All plants are home to a wide variety of microorganism communities including bacteria, fungi, and yeasts. Some microorganisms benefit the plant, others are plant pathogens and can potentially damage or kill the host plant (4). The majority of bacterial colonists on any given plant have no noticeable effect on plant growth or function.
Estimates suggest that the roughly 1 billion square kilometers of worldwide leaf surfaces host more than 10^26 bacteria, which are the most abundant colonizers of this habitat. Overall, microbiota in this ecosystem are large enough to have an impact on both global carbon and nitrogen cycles. Further more the phyllosphere mircroorganisms influence their hosts at the level of the individual plants.
With repeated and rapid constant change of environmental conditions occurring on leaf surfaces, the phyllosphere is recognized as a hostile environment to bacteria. Leaf surfaces are often dry and temperatures can reach 40–55°C under intense sunlight. During the night, however, leaves are frequently wet with dew and at cool temperatures (5–10°C) (2).
Microbes that live in the Phyllosphere are called [Epiphytes]
Physical environment
Distribution of Phages
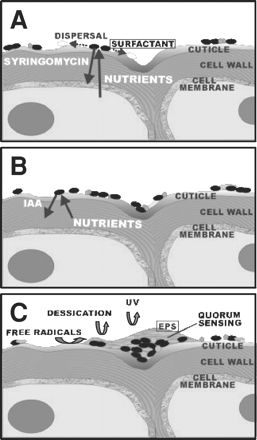
The leaf surface is exposed to rapidly changing temperature and relative humidity. Also the repeated alternation between presence and absence of moisture due to rain and dew (3). The leaf also provides limited nutrient resources to bacterial colonists. The rapid and severe changes in the physical conditions of the phyllosphere creates a hostile microbe environment.
Several factors may influence the microhabitat experienced by bacteria on leaves. First, the leaf itself is surrounded by a very thin laminar layer in which moisture emitted through stomata may be sequestered, alleviating the water that stress epiphytes (4).
Bacteria to Phage Ratio
Second, some cells in a leaf bacterial population, particularly in [plant-pathogenic] populations locally invade the interior of the leaf, avoiding the stresses on the exterior of the leaf by residing in substomatal chambers or other interior locations. While some phytopathogens have the option of avoiding stresses, most epiphytes must tolerate them in some way (Lindow, S).
Epidermal cells produce hills and valleys that will determine the shape and size of low areas on the surface, which will influence the shape and spread of water droplets on the plant. The first contact between immigrating bacteria and a leaf normally occurs at the plant cuticle. The waxy layer, which has different three-dimensional crystalline structures on different plant species and can change as leaves age. These modifications limit passive diffusion of nutrients and water vapor from the plant's interior onto the surface and defines the hydrophobicity of the leaf. Thick waxy cuticles have thus been thought to interfere with bacterial colonization of plants by limiting diffusion of nutrients and inhibiting the wetting of the leaf surface (Lindow, S).
Interaction Surfaces
Because the Phyllosphere is a hostile environment for the residing microorganisms physical parameters contribute to stressful conditions, such as UV radiation, temperature shifts, and the presence of reactive oxygen species. Adaptation to stressful conditions was reflected by the detection of various proteins, assigned to diverse bacterial genera and detected in all analyzed samples. Among these proteins were superoxide dismutase, catalase, DNA protection proteins, chaperones, and proteins involved in the formation of the osmoprotectant trehalose (1).
Applications in the Food Industry
Treatment Methods
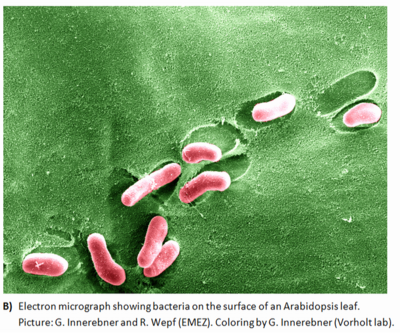
There is evidence that bacteria form large and heterogeneous aggregates on plant surfaces. Microscopic examinations of colonized leaves show that plant surfaces have many epiphytes occuring on them in large mixed-bacterial-species aggregates that also harbor fungi. While large numbers of solitary bacterial cells occur on plants, a few large masses of apparently mixed bacterial species can be found.
Such aggregates can constitute between 30 and 80% of the total bacterial population on certain plant species. These assemblages, with an extent and structure similar to those of biofilms that develop in aquatic habitats, are probably found only on long-lived leaves in moist climates such as the tropics or wet temperate regions such as the Pacific Northwest.
Benefits
The conglomerates on most other plants, while still sizable, are best known as aggregates. The formation of aggregates by bacteria on plants has major implications for the ability of these microbes to colonize and survive the harsh environment of the phyllosphere (1); it may provide them with a means to modify their immediate environment in the habitat . The production of extracellular polysaccharide (EPS), which is considered to form a major part of the bacterial aggregate matrix, may benefit epiphytes in the phyllosphere to see a photo of phyllospheric bacteria click the link microbial interactions
Safety
Water availability is one of the most highly fluctuating factors on leaf surfaces. The heavy EPS slime within aggregates can shield the bacteria from desiccation stress by buffering the matric and osmotic potentials of their surroundings. Furthermore, EPS helps to protect plant-associated bacteria from reactive oxygen species, which are often encountered on plants. It has been demonstrated that aggregated bacteria resist oxidative stress better than planktonic bacteria (1).
Key Microorganisms
E. coli
Certain bacteria in the Phyllosphere can increase the saturation of leaves via production of compounds with surfactant properties. This ability occurred in 50% of the Pseudomonas strains tested. Because of the hydrophobic nature of the cuticle, it is likely that increased saturation of these habitats allows diffusion of substrates, making them more readily available to epiphytic bacteria (Lindow, S). Biosurfactants facilitate the moving of bacteria on the phylloplane, this phenomena was suggested for tolaasin, a toxin produced by Pseudomonas tolaasi. The water film created by the surfactant could spread the bacteria across the leaf surface to areas where nutrients are more abundant. The production of biosurfactants may be one trait which bacteria can alter their habitat to exploit it more efficiently (3).
Listeria monocytogenes
Salmonella
Communities in the phyllosphere are thought to be limited by carbon availability, and it may be expected that access to carbon compounds on leaves is a major determinant of epiphytic colonization. There is evidence that small amounts of nutrients, such as simple sugars (glucose, fructose, and sucrose) leach from the interior of the plant. (1).
Current Research
Bacterial Resistance to Phage Therapy
Bacteria and their bacteriophage are constantly co-evolving. A study showed that E. coli O157, when incubated with phage PP01 for 200 hours, developed a series of mutants which differed in colony morphology, nature of phage receptors OmpC and LPS, and phage susceptibility [7]. The phage responded by evolving a broadened host range [7]. A trade off was observed between resistance to phage and competitiveness with parental strains for resources. For phage resistant strains to be selected for in the wild, they must also compete with many other strains that do not feel this phage pressure (unlike competing again only the phage-susceptible ancestor in the laboratory) [7]. If phage selective pressure is low, such mutants cannot be expected to present any danger in long-term phage based intervention (1). Depending on the phage however, many bacteria are favoured in this co-evolutionary arms race (some resistance in certain strains even come without a metabolic cost) [7], thus bacterial resistance may still pose to be a problem in the future.
Commercial Production of Phages
In order for phages to be effective in phage-mediated biocontrol, studies must be tested under conditions which resemble commercial practices. For zoonotic bacteria such as Salmonella, there is need to determine the optimal timing and delivery of bacteriophage in a real-life poultry industry setting [3]. In order to have this intervention be scaled up for commercial production, cost-effectiveness vs. efficacy in real-life application will need to be assessed (1). Market acceptance by the food industry and the consumer will need to occur before it can be considered an ideal antibacterial agent (1).
References
(1) Hagens, S., Loessner, M.J. “Bacteriophage for Biocontrol of Foodborne Pathogens: Calculations and Considerations.” Current Pharmaceutical Biotechnology 2010, 11, 58-68
(2) Delmonte, N., Knief, C., Chaffron, S., 2009. "Community proteogenomics reveals insights into the physiology of phyllosphere." National Academy of Sciences.
(3) Machowicz-Stefaniak, Z., Krol, E. 2006. "Biotic effect of caraway phyllosphere fungi on the pathogenic fungus." Department of Plant Pathology University of Life Science. 2-8
(4)Suslow, T. 2005. "Microbial Food Safety IS Your Responsibility." Vegetable Research Information Center. 1-6.
(5) Howplantswork. 2009. "Life in the Phyllosphere: What Microbes Commonly Dwell on the Surface of Leaves?."
(6) Whipps, J.M. Hand, P. Pink, D. 2008. "Phyllosphere microbiology with special reference to diversity and plant genotype." 2-34
(7) Gardener, B., Frafel, D. 2002. "Biological Control of Plant Pathogens: Research, Commercialization, and Application in the USA." Plant Health Progress. 1-18
(8) Abell, G., Richter, A. 2008. "Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica." The IMSE journal. 2, 561–570
Edited by student of Angela Kent at the University of Illinois at Urbana-Champaign.