Plant endophyte
Introduction
At the most basic level, endophyte simply means the location of an organism, with “endo” means “inside” and “phyte” means “plants”. Therefore, endophyte refers to organisms that live within plants (Wilson, 1995)[#Reference |[10]]]. Fungi and bacteria are the most common organisms associated with the term endophyte.
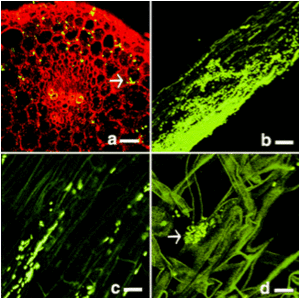
The photo on the right is showing enodphytic bacteria harbor within alfalfa. Every light spots on the photo represent a endophytic bacterial group.
Ecological significance
Endophytic organisms associated with plants are varied and complex. Endophytic microbes occupy a relatively privileged niche within plant and usually contribute to plant health. Some groups of endophytic microorganisms have been believed to be mutualists that protect plants against biotic stresses. Co-evolution may exist between endophytes and their host in resist to environmental stresses. During the last two decades endophytes have been targeted as valuable sources of new bioactive compounds (Tadych, & White, 2009)[15].
Importance of the microorganism in this habitat
Fungi, bacteria and various kinds of microorganism are found as endophytes. Some of the endophytes are proved to be able to enhance plant growth by nitrogen fixation[1], increase resistant against pathogens, remove contaminants and solubilize phosphate. Some bacterial endophytes are originally from the phyllosphere bacterial communities in [2], phyllophane, endophyte infected seeds and plant material.
Physical environment
Endophytic microorganisms depend on the nutrient supplied by host plants, so parameters affect plant nutrient supplies will consequently influent endophytic communities. Thus physical factors, such as temperature, rainfall, edaphic factors and UV radiation will affect endophytic communities indirectly. Those factors will influence the microorganisms from rhizophere and phylloplane in a similar way.
In addition, soil physical and chemical factors also have an indirect effect on the endophytic communities. The factors, including pH, salinity and soil texture can alter the saprophytic bacteria in rhizosphere, resulting in preselecting the endophytic bacterial source.
Microbial Community
Fungi
Endophytic fungi could be broadly defined as fungi that live for all, or at least significant part of their life cycle internally and asymptomically inside plants. Fungi are the most frequently isolated endophytes.
Endophytic fungi are very common and with high diversity living within plant tissue. Every plant species is found to be at least host one fungal endophytes, but usually asymptomatic and sometimes systemically (Faeth & Fagan, 2002)[2]. As endophytes, they usually occupy the above ground plant tissue, which distinguished them from mycorrhizal symbionts.
Bacteria
Endophytic bacteria are defined as bacteria that are detected “from inside surface-disinfested plants or extracted from inside plants and have no visibly harmful effects on the plants”extracted from inside plants and have no visibly harmful effects on the plants”(Hallmann, QuadtHallmann, Mahaffee, & Kloepper, 1997)[12]].
Endophytic bacteria, along with rhizospheric bacteria contribute to plant growth. And it is not yet clear which of these two bacteria contributes more to plant. Inside the plant tissue, the density of endophytic bacteria is less than rhizospheric bacteria and bacterial pathogens[3]. Endophytic bacteria are originally evolved from epiphytic bacterial communities in rhizosphere, phyllophane. From a phylogenetic view, endophytic bacteria are between saprophytic bacteria and plant pathogens. Overall, both biotic and abiotic effects influence the dynamic patterns of bacterial endophytes, the influences come especially from the host plants(Rosenblueth & Martinez-Romero, 2006)[7].
Biological interactions
Every plant found so far associate with at least one kind of endophytic microbes. Variety of interactions are going on ranging from microbe-host interaction to microbe-microbe interaction. Endophytes colonizing inside plant tissues contribute to the fitness of host and in return, they gain nutrient and protection from the host.
Fungi-Host Interaction
Endophytic fungi can be found within the leaves and stems of plants, with remarkable diversity. Endophytic fungi are traditionally thought to interact with host mutualistically. Systemic endophytic fungi are able to produce physiologically active alkaloids in host plant tissues. Thus, some agronomic species infected with systemic endophytes show resistance to toxic and noxious effects on vertebrate and invertebrate herbivores, as well as increase the host grass competitive abilities.
However, only minority endophytic fungi bring those benefits. Majority fungal endophytes, especially those horizontal transmitted one hosting in woody plants, do not show obvious or show even no impact to the host growth and resistance.
Bacteria-Host Interaction
Endophytic bacteria living with in plant tissue as biotrophic symbionts and these bacteria can be either obligate or facultative. Some endophytic bacteria are able to colonize thousands of different plant species, while some are restricted to plant families (bacteria endophyte, niche occupants and utility).
Endophytic bacteria produce a wide range of phytohormones, such as auxins, cytokinins, and the gibberellins. In addition, endophytic bacteria help to enhance the nutrient ability and fix nitrogen for plants. For instance, plant hormones produced by endophytic bacteria seem to be necessary for bryophyte development (Hornschuh, Grotha, & Kutschera, 2002) [13].
Endophytes-pathogen Interactions
Endophytic bacteria and fungi coexist with many other microorganisms, such as virus, other bacteria and fungi.
In the past, rhizosphere bacteria have been observed to be effective antagonists against many different sorts of fungal pathogens. Recently, studies have indicated that bacteria colonizing the plant interior are able to improve plant growth and suppress pathogen. Competitions between endophytic bacteria and pathogens are observed due to the limited nutrient supply inside plant tissue. Both endophytic fungi and bacteria produce antibiotic agents.
Although the facts of endophytic bacteria reducing the negative impact of plant pathogen have been well documented, few reports indicate plant pathogens have significant influence endophytic bacteria.
Natural Products of Endophytes
The bioactive natural products from endophytes are promising resources for medicine, agriculture and industry(Guo, Wang, Sun, & Tang, 2008)[3].
Different kinds of Alkaloids are contributed to plant by endophytes. Some of these alkaloids raise plants’ resistance to environmental stress, and some are growth-promoting compounds. Amines and amides are very common metabolite product from endophytes and have shown to be toxic to insect but not to mammals. Indole-3-acetic acid is a well-known phytohormone, which also can be produced by endophytes. Other bioactive compounds, such as steroids, terpenoids and diterpenes also are generated by endophytes (Tan & Zou, 2001)[8].
Besides the compounds mentioned above, endophytes produce extracellular hydrolyases in order to establish a resistance mechanism against plant invasion. The enzymes include cellulases, lipases, proteinase and esterases. The actions of those enzymes support the hypothesis of co-evolution between endophytes and their hosts(Tan & Zou, 2001)[8].
Key Microorganisms
Endophytic N2-fixing bacteria
Early evidence for non-symbiotic nitrogen fixation was provided by studies of N balance in various ecological studies. Different crops and grasses, such as sugar cane in Brazil, wetland rice in Asia, and some cereal field in Canada, were observed growing healthy without artificial nitrogen input. Those plants were thought to benefit from the endophytic nitrogen fixers. Thus, the nitrogen fixation bacteria associated with non-legume plant caught people’s attention.
The ecological and economical importance of nitrogen fixation in rhizobium-legume symbiosis (e.g. bradyrhizobium and soybean) has earned research attention for a long time. In legumes, the rhizobium stimulates the plants to develop root nodules. In root nodules, the bacteria infect, inhabit, and construct a co-metabolic system with the legumes, so as to form a well-developed symbiosis. Rhizobia inside root nodules fix nitrogen into ammonia, and export to the leguminous plants(Long, 1989)[6]. This process is highly efficient and provides significant proportion of plant nitrogen.
Recent work has proved that significant N fixation processes also exist in some non-legumes, particularly sugar cane, rice and maize, among which maize and sugar cane are well-known bio-fuel plants. Different from those well-known legume associated N-fixers, N-fixer associated with crops do not form species structure, and those detected bacteria usually form a different class. In addition, the nitrogen fixation rate is relatively lower comparing to legumes. The performances of N-fixers have been estimated in those crops, and the result varies according to different plant species(James, 2000)[4]. The highest fixation efficiency association so far found is a sugar cane species in Brazil and its symbiotic bacteria community by Döbereiner, Boddey, and coworkers (Urquiaga, Cruz, & Boddey, 1992)[9] . According to their work, the symbiotic nitrogen-fixer can fixed up to 70% N-requirement for the sugar cane. But more often, the performance of nitrogen fixation rate carried out by bacteria is not as satisfying as the example above. Even for sugar cane, only the one species found in Brazil associate with such high fixation efficient microbial community(James, 2000)[4]. Finding and “creating” new nitrogen fixing bacteria through gene engineering ares the new hot spot in this field.
Current Research
1. Endophytic nitrogen fixing bacteria enhance the growth of biofuel plants
The previous work on nitrogen-fixing bacteria in other grasses offers sufficient theoretical and methodological background for the further research on bio-fuel plants. Miscanthus and switch grass are two of the most promising bio-fuel plants have been plant widely in Europe and United States(Lewandowski, Scurlock, Lindvall, & Christou, 2003)[5]. However, little is known about the nitrogen-fixing bacteria associated with them. For future research, the nitrogen fixation bacteria associated with those candidate bio-fuel plants need to be identified, and it is also essential to evaluate their performance in plant.
2. Use the natural product of endophytic fungi antibiotic agent
As mentioned above, endophytes produce compounds against insect and plant pathogen. This dual biological control has been reported for the fungal entomopathogens, Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota: Hypocreales) and Lecanicillium spp. (Ascomycota: Hypocreales). However, the mechanisms under those processes are not yet clear. Generally, the mechanism of antibiosis includes production of antibiotic compounds, bioactive volatile organic compounds (VOCs) and enzymes. For further studies, the roles of endophytic fungi in suppression of diseases need to be understood, and the plant-microbe interactions are worth attentions too (Ownley, Gwinn, & Vega, 2010).
3. Rice endophyte metagenome
Nitrogen fixing bacteria have great potential in agricultural system. Better understand of mechanism is in need for wider application. Azoarcus sp. strain BH72 is a nitrogen fixer that has been found associated with sugarcane and Kallar grass. This strain is an excellent model for studies of endophyte - plant interaction. The complete genome sequence of Azoarcus sp. strain BH72 has been obtained through genome shotgun approach by Krause and his coworkers (Krause et al., 2007)[11]. The complete genome sequence is 4,376,040-bp long and contains 3,992 predicted protein-coding sequences. The complete genome sequence of Azoarcus sp. strain BH72 give insight into research about the endophytic life style of nitrogen fixation endophytes.
References
[9]Urquiaga, S., Cruz, K. H. S., & Boddey, R. M. (1992). CONTRIBUTION OF NITROGEN-FIXATION TO SUGAR-CANE - N-15 AND NITROGEN-BALANCE ESTIMATES. Soil Science Society of America Journal, 56(1), 105-114.
[14]Ownley, B. H., Gwinn, K. D., & Vega, F. E. (2010). Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. [Review. Biocontrol, 55(1), 113-128.]
[15]Tadych, M. and White, J. F. (2009). Endophytic Microbes, pages 431-442.
Edited by student of Angela Kent at the University of Illinois at Urbana-Champaign.
