Plasmodium falciparum
Plasmodium falciparum is a protozoan parasite that causes an infectious disease know as malaria. P. falciparum is the most severe of the malaria species correlated with almost every malarial death.[1] The other 3 species that cause malaria include P. vivax, P. ovale, and P. malariae. Humans become infected by a female Anopheles mosquito which transfers a parasitic vector through its saliva into the blood stream. The parasite then infects the liver and undergoes asexual reproduction followed by insertion into red blood cells where an additional round of replication takes place. P. falciparum changes the surface of an infected red blood cell causing it to stick to blood vessels, cytoadherence, as well as to other red blood cells.[2] In severe cases this leads to obstructions of microcirculation resulting in dysfunction of many organs. Symptoms depend on severity of infection and can present a range of signs such as flulike symptoms, vomiting diarrhea, shock, kidney failure, coma, and death. Plasmodium falciparum mostly infects children under the age of 5 as well as pregnant women. With treatment of antimalarials the death rate drops drastically from almost 100% to 20%.[3]
Etiology/Bacteriology
Taxonomy
| Domain = Eukarya | Kingdom = Chromalveolata | Phylum = Apicomplexa | Class = Aconoidasida | Order = Haemosporida | Family = Plasmodiidae | Genus = Plasmodium | Species = P. falciparum
Pathogenesis
Transmission

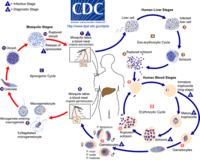
Transmission of P. falciparum occurs between humans and Anopheles mosquitos. Malaria is passed by mosquito vectors from host to host. The parasite can infect the mosquitos through the in take of human blood or a human by the mosquitos injection of saliva into the human. [1] Once the mosquito becomes infected with Plasmodium falciparum it transfers the disease to each new host it penetrates. Humans can rarely transfer the parasite between each other. There have been rare cases of contaminated transfused blood infecting the recipient, but seldom does this occur because of screening that takes place pre-blood donation. [4] Mothers can also pass P. falciparum to their child during birth, this is also a seldom occurrence.
Infectious Dose, Incubation, Colonization
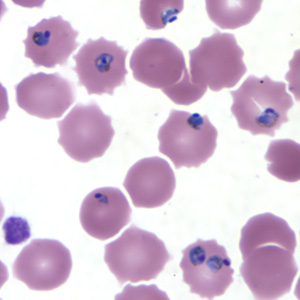
Symptoms of Malaria typically begin 8-25 days following infection, in few cases it can take up to a year. The late onset of incubation is due to taking an inadequate amount of anti-malaria medication.[5] The infectious dose is not precisely known, but it is understood to be a very low number. Malaria can be observed months to years after first set of symptoms are observed. This is due to the parasites ability to lie dormant in liver cells until the environment is right for a relapse. This is mainly seen in P.vivax and P. ovale, rather then P. falciparum.[1] The parasite colonizes in the liver and is then released into the blood stream and attached to erythrocytes.
Epidemiology
The key to Malaria-endemic is Anopheles mosquitos ability to live in an area. Temperature is also important having to stay above 20 degrees Celsius. The main areas of P. falciparum are South America, Africa, India, and few parts of Indonesia. The best possible location is along the equator in a warmer region. Transmission will not occur in high altitudes, colder seasons, and deserts.[1] Malaria is thought to have been around since the beginning of mankind, but was first discovered in blood in 1880 and found to be transmitted by mosquitos in 1889.[3] There are four common species of Malaria of which P. falciparum is the most severe. Plasmodium falciparum continues to increase in drug-resistant populations and insecticide-resistant mosquitos leading to the prediction that the disease will only worsen over time.
Virulence Factors
PfEMP1,P. falciparum erythrocye membrane protein 1, is an adhesive ligand protein which is created inside of a P. falciparum infected erythrocyte and presented on the surface. PfEMP1 is known as a knob and is encoded by the multigene segment, Var. The protein is responsible for sequestration within the vital organs. In some case were sequestration occurs in the brain this will lead to the cerebral form of malaria. Each Plasmodium falciparum has multiple versions of PfEMP1 with which it can alter its appearance by changing to another PfEMP1 when the immune system begins to create antibodies for the original PfEMP1 in a process known as antigenic variation.[6]Changing of adherence molecules also means a change in the receptor on the epithelial. The change in receptor is hypothesized to possible change the disease outcome.
RIFIN, repetitive interspersed family, is considered the most abundant multigene family. PfEMP1 along with RIFIN is considered a crucial cornerstones for the virulence of Plasmodium falciparum mainly due to its ability to avoid immune response through antigenic variability and ultimately colonizing and replicating in the liver and erythrocytes.[6] RIFIN is also presented on the outer membrane of an parasite infected erythrocye as an adherence factor.
Rosettes are uninfected red blood cells that form clumps with Malaria-infected erythrocytes. Clumping occurs when particularly sticky PfEMP1 attach to other red blood cells. Only a minority of P. falciparum actually creates rosettes, but when they do they are known to be linked to severe malaria.[7]
Malaria pigment (hemozoin) is released during erythrocyte rupture. Cause the uncomplicated symptoms of malaria such as chills and fever.[8]
Life Cycle
The life cycle of malaria is a complex process infecting two hosts, human and mosquito. The process begins when a infected mosquito transfering saliva as well as sporozoites into an individuals circulatory system. These sporozoites travel to the liver and invade hepatocytes. In the liver asexual reproduction occurs through exoerythrocytic schizogony, which produces merozoites that are released back into the blood. From here the merozoites invade an erythrocytes and begin the trophic period. During this period the trophozite enlarge followed buy multiple rounds of asexual nuclear division to a schizont. Merozoites bud from the schizont and eventually rupture the erythrocyte releasing toxins that cause the simple symptoms of malaria, fever and chills. Merozoites eventually invade another erythrocyte which begins another round of the blood stage replication. Some erythrocytes change into gametocytes capable of doing sexual reproduction. These cells do not lyse but instead are taken up but the next mosquito that bites, infecting the mosquito and possible more people.[9]
In severe cases caused by P. falciparum symptoms can be vastly more complicated leading to coma and possibly death. This is due to trophoziote and schizout be sequestered into deep tissue which can limit oxygen to other areas of the body.
Sickle Cell Resistance
Sickle cell individuals have shown to rarely contract malaria. Research has shown that this is partially due to weakened binding of parasite infested sickle cell erythrocytes to microvasculare endothelial cells when compared to normal hemoglobin parasite erythrocytes binding. The virulence factor PfEMP1 that normally conducts cytoadherence is altered creating a weekend attachment between it and the epithelial wall. Due to the ability to attach lacking, sequestration would also not occur limiting the severe malarial response. The mechanism for how this is done is still unknown and needs further research.[10]
Clinical features
Symptoms
P. falciparum normally proceeds in two forms, uncomplicated or severe. In most occurrence the severe case is observed showing symptoms such as cerebral malaria, which cause abnormal behavior, seizures, coma, or impairment of consciousness. Severe symptoms also present anemia due to destruction of red blood cells, hemoglobinuria, acute respiratory distress, low blood pressure, acute kidney failure, metabolic acidosis, and hypoglycemia. These are all due to organ failure and abnormalities in patient's blood or metabolism.
During a rare uncomplicated infection, symptoms appear flu-like. The attack lasts roughly 6-10 hours presenting a cold stage, hot stage, and sweat stage. During these stages one shows symptoms of fever, chills, sweats, headache, nausea, vomiting, body ached, and malaise.[1]
Morbidity and Mortality
In 2010 malaria was diagnosed for 219 million people and killed 660,000 people. Roughly 70% being 5 years or younger and 75% of these cases were caused by P. falciparum. [1]
Pregnant women are at higher risk for a more severe reaction to themselves and the fetus. In some cases malaria my cause prematurity, abortion, and stillbirth.
Young children are also at higher risk for more severe infections due to the immaturity of their immune systems.
Diagnosis
Rapid and accurate diagnosis using microscopic examination of blood smears is the most precise way to determine Plasmodium falciparum as the disease. CDC provides various references for microscope diagnosis along with serology, PCR, and drug resistance testing. Each species of P. falciparum has distinctive characteristics that can be see under a microscope. In only early form, trophozites and gametocytes of P.falciparumare seen in the blood as ring form inside the erythrocyte. There are normally multiple parasites in one erythrocytes appearing as several dots. [1]
Treatment
The best line of defense against any form of malaria is preventative treatment, antimalarial, taken properly before, during, and after exposure to parasite.
Treatment of P. falciparum depends on severity of infection as well as location where the infection took place. Treatment can also vary due to an individuals age, weight, and pregnancy status.
In uncomplicated malaria, the first line of defense includes Artemisinin-based combination therapy (ACT). ACTs are used to improve treatment by overcoming the resistance by using more then one derivative of Artemisinin. The choice of which ACT to use depends on the region in which the infection took place. This is due to the varying level of resistance found in different areas. Non-ACTs such as sulfadoxine-pyrimethamin with chloroquine can also be used but are considered to have a limited sufficiency due to drug resistance.[11]
In severe malaria, the main focus is to keep the patient from dying. Rapid clinical assessment and confirmation are key.
Prevention
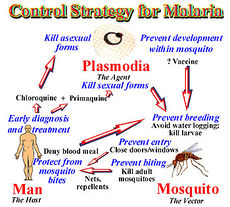
Risk Avoidance
In 1992, the W.H.O. redirected the strategy towards malaria from vector control to treatment. The control of malaria entails 3 living beings: human, mosquito, and vector. Each has its own complications and if treated properly ability to stop the cycle of malaria. For successful malaria control it is now believe to target man first, mosquitoes next followed by the parasite. The web of interact allows the control of malaria on one of these systems to complement the others.[11]
Immunization
Studies are still on going for an immunization for Plasmodium falciparum. The prospects of a vaccine do not look promising due to infected individuals never developing sterilizing immunity. The parasite has an impressive ability to avoid and suppress the immune system never allowing it to create the proper antibodies to fight the infection. [3]
Host Immune Response
A key feature to the virulence of Plasmodium falciparum is antigenic diversity. This is the parasites ability to switch erythrocyte- associated antigens, thus evading the immune system. The alteration between antibodies only occurs in the trophozoite/schizont stage in the erythrocyte. The switch in erythrocites surface antigens as well as multiple strains of P. falciparum have prevented the creation of a vaccine. Plasmodium falciparum's clever avoidance of the spleen and immune system has created a parasite almost impossible to eradicate from the host without anti-malaria medications. [12]
References
1 Centers for Disease Control and Prevention (2012), Malaria <http://www.cdc.gov/malaria/about/disease.html>.
2 Cross, Caroline. "Welcome Trust." Malaria, Plasmodium flaciparum. N.p., 08 11 2004. Web. 24 Jul 2013. <http://malaria.wellcome.ac.uk.>.
3 Davis, Charles. "Medicine Net On Health." Malaria. William shiel. Web. 24 Jul 2013. <http://www.onhealth.com/malaria/article.htm>.
4 Vareil M-O, Tandonnet O, Chemoul A, Bogreau H, Saint-Léger M, Micheau M, et al. Unusual transmission of Plasmodium falciparum, Bordeaux, France, 2009. Emerg Infect Dis [CDC Emerging Infectious Disease]. 2011 Feb [07/24/2013]. <http://wwwnc.cdc.gov/eid/article/17/2/10-0595_article.htm>.
5 Nadjm B, Behrens RH (2012). "Malaria: An update for physicians". Infectious Disease Clinics of North America 26 (2): 243–59. <https://www.ncbi.nlm.nih.gov/pubmed/22632637>.
6 Bultrini, Emanuele . "Revisiting the Plasmodium falciparum RIFIN family: from comparative genomics to 3D-model prediction." BMC Genomics. N.p., 20 09 2009. Web. 24 Jul 2013. <http://www.biomedcentral.com/1471-2164/10/445>.
7 Ho M, Davis TME, Silamut K, Bunnang D, White NJ. Rosette formation of Plasmodium falciparum-infected erythrocytes from patients with acute malaria. Infect Immun. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC257977/>
8 Ogobara K. Doumbo, et al. "The Pathogenic Basis Of Malaria." Nature 415.6872 (2002): 673. Academic Search Premier. Web. 24 July 2013.
9 Wiser, . "Plasmodium Life Cycle." . N.p.. Web. 24 Jul 2013. <http://www.tulane.edu/~wiser/malaria/mal_lc.PDF>.
10 Rick M Fairhurst, et al. "Impaired Cytoadherence Of Plasmodium Falciparum-Infected Erythrocytes Containing Sickle Hemoglobin." Proceedings Of The National Academy Of Sciences Of The United States Of America 105.3 (2008): 991-996. MEDLINE. Web. 24 July 2013.
11 Kakkilaya, . "Treatment of Malaria." Malaria Site. N.p., 16 05 2012. Web. 24 Jul 2013. <http://www.malariasite.com/malaria/Treatment4.htm>.
12 Hommel, M, P H David, and L D Oligino. "Surface Alterations Of Erythrocytes In Plasmodium Falciparum Malaria. Antigenic Variation, Antigenic Diversity, And The Role Of The Spleen." The Journal Of Experimental Medicine 157.4 (1983): 1137-1148. MEDLINE. Web. 24 July 2013.
Created by {Kelley Raines}, students of Tyrrell Conway at the University of Oklahoma.
