Poliomyelitis

| Poliovirus | |
|---|---|
| Scientific classification | |
| Group: | Group IV |
| Order: | Picornavirales |
| Family: | Picornaviridae |
| Genus: | Enterovirus |
| Species: | Enterovirus C |
| Subtype: | Poliovirus |
Description
Poliomyelitis is viral disease caused by an enterovirus known as poliovirus and is well known for its role in causing paralysis, especially in infants. While most infections are asymptomatic, viral particles that gain entrance into the central nervous system can replicate in neurons and destroy cells that govern muscle function resulting in flaccid paralysis.
Epidemics involving the disease, also known as polio, have stuck the human race throughout history. The emergence of polio vaccines in the mid 20th century, however, has given public health organizations the tools needed to eradicate the disease. Worldwide immunization efforts have reduced worldwide cases from the hundreds of thousands to less than a thousand per year. With the Western Hemisphere and Europe declared polio-free in 1994 and 2002 respectively, the virus only remains endemic in Nigeria, Afghanistan and Pakistan. If successfully eradicated, polio will be one of only three diseases eradicated in history—the others being smallpox and rinderpest which were declared eradicated in 1979 and 2011 respectively.
Virology and Taxonomy
Poliovirus is a member of the picornavirus viral family, a taxonomic grouping that includes rhino viruses and hepatisis A virus. Picornaviruses are characterized by their icosahedral capsid structure that lacks a viral envelope and carries the single-strand RNA genome. Enterovirus, the genus that includes poliovirus, represents viruses that can withstand low pH and thus pass through the stomach to infect and replicate within intestinal epithelial cells. This ability allows poliovirus to be highly infectious through the fecal-oral route.
Genome and Structure
The virus’ approximately 7500 nucleotide RNA genome codes for the proteins that form its capsid as well as _______. As the genome is translated within the host cell, the polyprotein is cleaved to release P1, which codes for all the proteins needed to form the capsid. 3CD, a viral protease, cleaves P1 to release VP1, VP3 and myristoyl-VP0 which will form pentamers that will eventually create empty capsids made of 60 copies of each of the aforementioned proteins. Once the RNA genome has been encapsidated, the infectious virion will be formed with the cleavage of VP0 into VP2 and VP4 thus leaving the capsid structure complete with 60 copies each of the four proteins: VP1, VP2, VP3 and VP4.
Serotypes
Three distinct serotypes, PV1, PV2 and PV3, are associated with the paralytic disease with PV1 being associated most frequently with paralytic poliomyelitis.
Pathogenesis
Transmission
As a virus that is shed in feces even by asymptomatic persons, poliovirus passes from person to person through the fecal-oral route. Highly communicable, poliovirus tends to be passed to 90 percent of adults and 100 percent of children in a single household. The virus, however, is only infectious in humans. Upon infection, the virus will multiple in the oropharyngeal and intestinal mucosa before draining into the lymph nodes and into the blood. Most infections do not progress past this point and cause minor symptoms. A small amount of infections, however, do pass into the central nervous system and cause various degress of paralysis. Incubation times can vary depending on what symptoms emerge. Minor illnesses of the virus typically manifest themselves between three to five days while more serious symptoms related to CNS infection take one to two weeks.
Entering host cells
Poliovirus enters host cells by binding to its receptor identified as CD155, a glycoprotein of the immunoglobin superfamily. Once multiple virus particles bind the V-type domains of their receptors, they viral particles undergo a conformational change which externalizes the proteins VP4 and VP1 found on the capsid. The proteins then insert into the cell membrane forming channels in the cell membrane. *** The pathway by which the viral particles are internalized in the cell is throught to depend on actin and tyrosine kinase. Shortly after endocytosis of the virus particle, the RNA genome is quickly released into the cytoplasm through a pore in the newly formed vesicle. As genome release occurs within only 100 to 200 nm of the cell surface, the event might be triggered as the resulting curvature of the forming vesicle inserts more VP1 N-termini into the cell membrane.
Dissemination into central nervous system
While the mechanism by which poliovirus enters the CNS remains undefined, research suggests two possible routes: passage through the blood-brain barrier and transport through neural pathways. Antiviral antibodies given intravenously to mice prevents infection of the brain and spinal cord suggesting that the resulting viremia plays a role in paralytic cases. In addition, the virus has been shown to travel up nerve cells exceeding 12 cm a day, and the molecular mechanism is thought to involve Tctex-1, a part of the retrograde motor complex. Upon entry into the cell, the vesicle containing the virus particle is thought to be connected to Tctex-1 by the cytoplasmic domain of CD155. This interaction allows the vesicle to be transported toward the cell body of the neuron where the RNA genome is released for replication. The process is repeated through each nerve cell to allow the virus to gradually progress toward the spinal cord and then the brain.
Clinical features
Poliovirus infection causes a wide range of symptoms depending partly on how far the infection progresses. About 93 percent of infections are asymptomatic, but infected persons still shed the virus in their stool allowing the virus to infect others.
Minor illness associated with poliovirus
Abortive poliomyelitis constitutes up to 5 percent of infections and presents itself with symptoms ranging from fever to gastrointestinal symptoms up to a week. Considering the common set of symptoms, abortive poliomyelitis cannot be distinguished from other viral infections.
Stiffness in muscles of the back and legs is associated with non-paralytic poliomyelitis and occurs in 1 to 2 percent of infections. As with all of the aforementioned syndromes, complete recover follows between one to two weeks.
Paralytic poliomyelitis
Paralytic poliomyelitis, occurring in about 1 percent of infections, accounts for the flaccid paralysis for which poliovirus is well-known. Paralytic symptoms manifest themselves anywhere from one to ten days after symptoms of the minor illness appear. Muscle pain and loss of superficial reflexes can accompany the progression of the paralysis, which typically affects the lower limbs. Nerves controlling the diaphragm can deteriorate upon infection leaving the patient unable to breath without mechanical assistance, historically in the form of an iron lung. Many infected persons recover most motor function after the infection has passed, but paralysis remaining after one year becomes permanent.
Paralytic poliomyelitis comes in three categories: spinal, bulbar and bulbospinal, a mixture of the two. Spinal polio accounts for 79 percent of paralytic cases and constitutes the progression of flaccid paralysis previously described and involves invasion of grey matter in the spinal cord. The resulting destruction of anterior horn cells leaves muscle fibers without a way to receive motor nerve signals from the brain. In two percent of paralytic cases, infection damages cranial nerves and the reticular formation in the brain stem resulting in a variety of problems: inability to spit, dysphagia, dysphonia, difficulty in chewing and regurgitation of food. Known as bulbar polio, the extensive brain damage contributes to the mortality associated with this category of infection. The remaining 19 percent of paralytic cases are considered to be bulbarspinal polio and result from infection of the upper cervical spinal cord. Cell necrosis in this area damages the phrenic nerve controlling the diaphragm thus leaving the patient unable to breathe on his or her own.
Post polio syndrome
Clusters of symptoms observed anywhere between 7 to 71 years after an initial poliovirus infection have been described in those who survive paralytic poliomyelitis. Collectively termed “Post-Polio Syndrome,” the symptoms include many of the same symptoms viewed in acute polio infection such as increased muscle pain, general fatigue, and loss of function unrelated to other medical conditions. Since some such symptoms can be common for aging patients, there is some disagreement about the definition of PPS as a separate health problem.
One of the more serious complications of post-polio syndrome is the emergence of respiratory problems that affect a person’s ability to clear mucosal secretions and contribute to the sleep apneas observed in people with post-polio syndrome.
Diagnosis
Since little can be done to prevent the progress of the virus upon infection, diagnosis usually provides little benefit to the patient. Furthermore, more than 95 percent of infections are either asymptomatic or indistinguishable from other viral infections, so a majority of cases are never diagnosed.
Laboratory diagnosis includes isolation of the virus and serotyping. If the virus is isolated from a patient exhibiting paralytic symptoms, PCR will be run to determine if the virus is of the wild-type or originated from an attenuated virus found in the polio vaccine. Determining the origin is of importance to public health organizations since one case of the wild-type virus can imply that up to 3000 asymptomatic carriers are still shedding the virus.
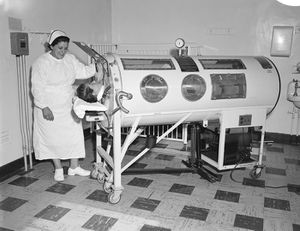
Treatment
Prevention
Host Immune Response
References
Created by Jake Morgan, student of Tyrrell Conway at the University of Oklahoma.
