Propionibacterium acne: pathogenesis in Acne Vulgaris: Difference between revisions
Caseyjinkx (talk | contribs) |
Caseyjinkx (talk | contribs) |
||
| Line 2: | Line 2: | ||
''Propionibacterium acnes'' belong to a larger human cutaneous (pertaining to the skin) family of <span class="plainlinks">[http://en.wikipedia.org/wiki/Propionibacterium ''Propionibacteria'']</span> [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1]]]. There is a normal benign presence of ''Propionibacteria'' in a healthy skin microflora and also manifest in the mouth and gut [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[2]]]. As gram- positive organisms, ''P. acnes'' bacteria possess a cell wall that provides high structural stability allowing resistance to drying, osmotic shock and mechanical stress [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1]]]. These organisms are also non-motile and non-spore-forming [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1,3]]]. As cutaneous microorganisms, ''P. acnes'' are <span class="plainlinks">[http://en.wikipedia.org/wiki/Primary_nutritional_groups chemoorganotrophic]</span>, requiring organic nutrients for oxidation to produce energy and cell biomass [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1]]]. | ''Propionibacterium acnes'' belong to a larger human cutaneous (pertaining to the skin) family of <span class="plainlinks">[http://en.wikipedia.org/wiki/Propionibacterium ''Propionibacteria'']</span> [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1]]]. There is a normal benign presence of ''Propionibacteria'' in a healthy skin microflora and also manifest in the mouth and gut [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[2]]]. As gram- positive organisms, ''P. acnes'' bacteria possess a cell wall that provides high structural stability allowing resistance to drying, osmotic shock and mechanical stress [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1]]]. These organisms are also non-motile and non-spore-forming [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1,3]]]. As cutaneous microorganisms, ''P. acnes'' are <span class="plainlinks">[http://en.wikipedia.org/wiki/Primary_nutritional_groups chemoorganotrophic]</span>, requiring organic nutrients for oxidation to produce energy and cell biomass [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[1]]]. | ||
== Metabolism == | |||
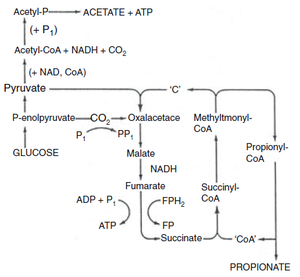
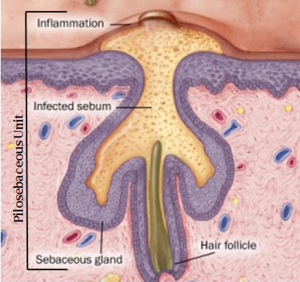
In the skin’s normal flora, aerotolerant anaerobic ''P. acnes'' grow in the pilosebaceous follicles [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[4]]] (Fig 2). [[File:Pacnefermentation.png|thumb|alt=Puzzle globe|Fig 1. Propionic acid fermentation forming propionate]] These bacteria can undergo an energetically favourable detoxification mechanism of denitrification through <span class="plainlinks">[http://en.wikipedia.org/wiki/Denitrification dissimilatory nitrate reduction]</span> [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[5]]]. This process is important since it involves energy conservation through the generation of a proton motive force or amplification of substrate-level phosphorylation reactions [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[5]]]. Under the anaerobic conditions of the follicles, ''P. acnes'' can undergo both fermentative energy conservation as well as anaerobic respiration [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[6]]]. Fermentation results in short chain fatty acids including propionic acid ( for which the bacteria is named after) and anaerobic respiration can involve nitrate reduction, glycerol-3- phosphate dehydrogenation and dimethyl sulfoxide reduction [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[6]]]. | In the skin’s normal flora, aerotolerant anaerobic ''P. acnes'' grow in the pilosebaceous follicles [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[4]]] (Fig 2). [[File:Pacnefermentation.png|thumb|alt=Puzzle globe|Fig 1. Propionic acid fermentation forming propionate]] These bacteria can undergo an energetically favourable detoxification mechanism of denitrification through <span class="plainlinks">[http://en.wikipedia.org/wiki/Denitrification dissimilatory nitrate reduction]</span> [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[5]]]. This process is important since it involves energy conservation through the generation of a proton motive force or amplification of substrate-level phosphorylation reactions [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[5]]]. Under the anaerobic conditions of the follicles, ''P. acnes'' can undergo both fermentative energy conservation as well as anaerobic respiration [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[6]]]. Fermentation results in short chain fatty acids including propionic acid ( for which the bacteria is named after) and anaerobic respiration can involve nitrate reduction, glycerol-3- phosphate dehydrogenation and dimethyl sulfoxide reduction [[Propionibacterium_acne:_pathogenesis_in_Acne_Vulgaris#References|[6]]]. | ||
Revision as of 06:52, 27 November 2013
General Characteristics
Propionibacterium acnes belong to a larger human cutaneous (pertaining to the skin) family of Propionibacteria [1]. There is a normal benign presence of Propionibacteria in a healthy skin microflora and also manifest in the mouth and gut [2]. As gram- positive organisms, P. acnes bacteria possess a cell wall that provides high structural stability allowing resistance to drying, osmotic shock and mechanical stress [1]. These organisms are also non-motile and non-spore-forming [1,3]. As cutaneous microorganisms, P. acnes are chemoorganotrophic, requiring organic nutrients for oxidation to produce energy and cell biomass [1].
Metabolism
In the skin’s normal flora, aerotolerant anaerobic P. acnes grow in the pilosebaceous follicles [4] (Fig 2).
These bacteria can undergo an energetically favourable detoxification mechanism of denitrification through dissimilatory nitrate reduction [5]. This process is important since it involves energy conservation through the generation of a proton motive force or amplification of substrate-level phosphorylation reactions [5]. Under the anaerobic conditions of the follicles, P. acnes can undergo both fermentative energy conservation as well as anaerobic respiration [6]. Fermentation results in short chain fatty acids including propionic acid ( for which the bacteria is named after) and anaerobic respiration can involve nitrate reduction, glycerol-3- phosphate dehydrogenation and dimethyl sulfoxide reduction [6].
Conditions of Pathogenicity
Acne Vulgaris is a disease of the epidermal pilosebaceous follicles involving inflammatory and non-inflammatory clinical lesions in the skin. The multifactorial pathogenesis of acne includes sebum, ductal epidermal hyperproliferation, colonization of Proprionibacterium acnes and inflammation [7]. Research has shown, P. acnes is not the cause of Acne Vulgaris, but is a significant contributing factor to the inflammation stage of this disease [8]. Sebum secretion upsurge, during times such as puberty, results in linoleic acid depletion in the follicles [1]. This affects the barrier function of the follicular walls and as a consequence results in the influx of water from the dermis into the lumen of the follicle, which is the site of colonization of Propionibacterium acne [1].
As the environment progressively becomes more anaerobic, colonization of follicles by microbes, including P. acnes is promoted [1]. This increase in P. acnes growth leads to pathogenicity involving the bacteria’s ability to produce bioactive exocellular products that directly damage host tissue and/ or induce inflammatory response through interactions with the immune system [9].
Pathogenic Activity through Toll- Like Receptor Interactions
Propionibacterium acnes, through interactions with Toll- like receptors 2 and 4, contribute to the inflammatory stage of acne by inducing monocytes to secrete pro-inflammatory cytokines such as TNF-α, IL-1β and IL-8 [7,9]. Similarly, P. acnes cell envelope surface molecules such as GroEL, heat shock proteins, DnaK (chaperon complex) and lipoglycans may all act as ligands for the TLR 2 or 4 on keratinocyte and sebocytes [7]. These interactions induce not only the production and secretion of cytokines by these epidermal cells, but P. acnes also activate differentiation and proliferation of keratinocytes [7]. MMPs are endopeptidases produced by keratinocytes that mediate the rupture of follicles resulting in epidermis acne lesions, as well as enhance inflammation [7]. Following this, augmentation of TLR 2 and 4 expressions occur [7]. A TLR induction and signal augmentation cycle is driven by this positive feedback which prolongs inflammation and pathogenesis.
Pathogenic Activity through Exoenzymes
Furthermore, oxygen tension in the follicles leads to porphyrin production by Propionibacterium acnes which can interact with molecular oxygen resulting in toxic oxygen species (free radicals) [4]. This can then cause damage to adjacent keratinocytes and contribute to cytokine release and problematic progression of the lesions [4]. As P. acnes causes hyperproliferation and resulting accumulation of keratinocytes, blockage in follicular duct and lumen may then ensue [4]. This, combined with the secretion of P. acnes in biofilm establishment on the follicular walls, results in the formation of microcomedone [7]. Cells within these biofilms exhibit altered properties,specifically pertaining to growth rate and gene transcription leading to enhanced TLR interactions [10,11]. As microcomedone (primary acne lesions) progress, a wide spectrum of P. acnes products and enzymes may cause microcomedonal inflammation [12]. Propionibacterial lipase hydrolyzes sebaceous triglycerides to produce free fatty acids [12]. As a main metabolic acid product, propionate (Fig 1) is an irritant and contributes to the severity of the acne inflammation [12]. Triggered by high bacterial cell density, P. acnes possess quorum sensing mechanisms through autocrine signal processes which upregulate extracellular enzyme production, such as lipase protease, hyaluronate lyase, and neuraminidase [4]. These excretions affect both the barrier function of the follicular wall as well as keratinocyte integrity [4].
Resistance in Treatment and Prolonging Disease
Propionibacterium acnes is able to induce inflammation through interactions with both the innate immune system and adaptive immune system leading to chronic inflammation. Population increase of P. acnes within the follicle increases immunogenic protein [8]. Cytokines and TLR 2 interactions will also direct T cell migration into the area, developing into prolonged inflammation due to escaladed release of immunological cytokines [8]. Research has found a high antibiotic resistance of Acne Vulgaris patients carrying P. acnes [3]. Ability to form biofilms, which gives rise to high resistant sessile P. acnes in the pilosebaceous units (Fig 2), is a contributing factor to treatment failure [3,10]. With the formation of the protective extracellular polysaccharide matrix dextran (glucan) by glucosyltransferase in these biofilms, antibiotic resistance is further enhanced [10].
References
- Bojar Richard A. and Holland Keith T. “Acne and Propionibacterium Acnes,” Clinics in Dermatology, no. 22 (2004): 375-379, accessed October 14, 2013. doi: 10.1016/j.clindermatol.2004.03.005.
- Cummins Cecil S., Johnson John L. and Stachebrandt Erko. “Family Propionibacteriaceae: The Genus Propioibacterium,” Procaryotes, no. 3 (2006): 400-418, accessed October 14, 2013. doi: 10.10070/0-387-300743-5_19.
- Coenye T., Honraet K., Nelis H.and Rossel B. “Biofilms in skin infections: Propionibacterium acnes and Acne Vulgaris,” Infectious Disorder Drug Targets, no. 3 (2008): 156-159, accessed October 14, 2013. http://web.ebscohost.com.ezproxy.library. ubc.ca/ehost/pdfviewer/pdfviewer?sid=126c8670-afda-4696-8989153e2fdeb41a%40 sessionm gr11&vid=2&hid=14.
- Aldana O., Bojar R., Cunliffe W., Eady E., Holland D., Holland K., Ingham E., McGeown C., Till A. and Walters C. “Propionibacterium acnes and Acne,” Dermatology, no. 196 (1998): 67-68, accessed October 14, 2013. http://BioMedNet.com/Karger
- Allison Clive and Macfarlane George T. “Dissimilatory Nitrate Reduction by Propionibacterium acnes,” Applied and Environmental Microbiology, no. 55 (1989): 2899-2903, accessed October 14, 2013. http://www.ncbi.nlm.nih.gov/pmc/articles /PMC 203188/?tool=pmcentrez& report=abstract.
- Bruggeman H., Durre P., Gottschalk G., Henne A., Hoster F., Hujer S., Liesegang H., Strittmatter A. and Wiezer A. “The complete genome sequence of Propionibacterium acnes a commensal of human skin,” Science, no. 305 (2004): 671-673 accessed October 14, 2013. http://www.jstor.org.ezproxy.library.ubc.ca/stable/3837368.
- Andreas D., Dessinioti C. and Katsambas M. “The role of Propionibacterium in acne pathogenesis: facts and controversies,” Clinics in Dermatology 28 (2010): 2-7, accessed October 14, 2013. doi: 10.1016/j.clindermatol.2009.03.012.
- Farrar M. and Ingham E. “Acne Inflammation,” Clinics in Dermatology, no. 22 (2004): 380-384, accessed October 14, 2013. doi:10.106/j.clindermatol.2004.03.2006.
- Lambert P. and Perry A. “Propionibacterium acnes,” Letters in Applied Microbiology, no. 42 (2006): 185-186, accessed October 14, 2013. doi: : 10.1111/j.1472-765X.2006.01866.x.
- Alexeyev O. and Jahns A. “Sampling and detection of skin Propionibacterium acne: Current status,” Anaerobe, no. 18 (2012): 479-483, accessed October 14, 2013. doi: 10.1016/j.anaerobe.2012.07.001.
- Dreno B., Jarrousse V., Jugeau S., Khammari A., Knol A. and Quereux G. “ Induction of toll-like receptors by Propionibacterium acnes,” Cutaneous Biology, no. 153 (2005): 1105-1113, accessed October 14, 2013. doi: 10.1111/j.1365-2133.2055.06933.
- Martin L. Muamba. “Exoenzymes of Propionibacterium acnes,” Canadian Journal of Microbiology 28 (1982): 758-761. doi: 10.1139/m82-115.