Pyrobaculum aerophilum: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
{{ | {{Biorealm Genus}} | ||
==Classification== | ==Classification== | ||
Revision as of 18:46, 15 May 2007
A Microbial Biorealm page on the genus Pyrobaculum aerophilum
Classification
Higher order taxa
Archaea; Crenarchaeota; Thermoprotei; Thermoproteales; Thermoproteaceae; Pyrobaculum
Species
Pyrobaculum aerophilum
|
NCBI: Taxonomy |
Description and significance
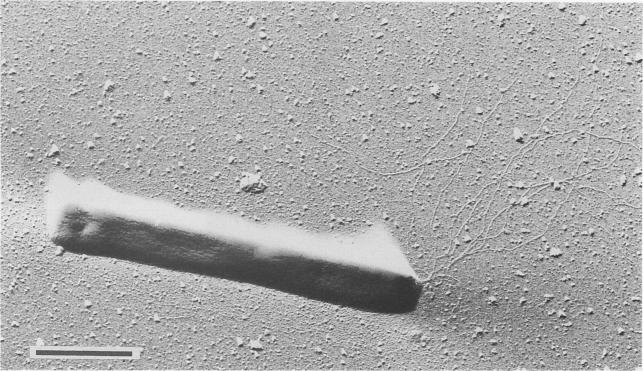
Pyrobaculum aerophilum is a rod-shaped hyperthermophilic archaeum that was first isolated from boiling marine water in Maronti Beach, Ischia, Italy. Pyrobaculum aerophilum derives its name from the Greek noun "aer" (air) and the Greek adjective "philos" (loving). It was found that the archaeum grew optimally at 100°C and at pH 7.0. Both organic and inorganic compounds served as substrates during aerobic and anaerobic respiration. However, growth was inhibited by elemental sulfur. When discovered, pyrobaculum aerophilum resembled members from the genera Thermoproteus and Pyrobaculum because of its ability to transform into spherical bodies, which resemble golf balls. After its 16S rRNA was sequenced, the new archaeum displayed traits more characteristic of the genus Pyrobaculum and was therefore classified as Pyrobaculum aerophilum. Most species in the genus Pyrobaculum cannot live in the presence of oxygen; however, the pyrobaculum aerophilum can interestinigly utilize oxygen for growth.
Pyrobaculum aerophilum cells were usually found to be 3 to 8 μm long and 0.6 μm wide. Very rarely, however, cells with a lengtht of 20 μm appeared. Motility is achieved using monopolar flagellation with bundles up to eight flagella. Each flagella averaged a diameter of 10 nm. Cells would transform into their terminal spherical phase mainly in the stationary-growth phase, at high nitrate concentrations, or pH values exceeding 8.0. Even in their terminal sphericacl phase, the cells can still enlarge or shrink depending on conditions. Colonies of Pyrobaculum aerophilum in their spherical phase exhibited greyish-yellow colonies with a harsh surface. Anaerobically condtioned cells in their normal rod-shaped phase exhibited a deep green color while aerobically grown cells in their normal rod-shaped phase exhibited a brownish yellow color.
Pyrobaculum aerophilum grew in mediums ranging from temperatures 75 and 104°C. At the optimum temperature of growth, 100°C, the optimal doubling rate of the cells was observed to be 180 minutes. At the extreme temperature range of 75°C, the doubling rate of the cells dropped to a rate of appoximiately 5 days. There was no difference in growth rate when the archaeum was cultured either in anaerobic and anaerobic conditions. When living conditions were observed in different pH conditions, life was observed from a range of pH 5.8 to 9.0. The optimum rate of growth was observed at pH 7.0. However, when pyrobaculum aerophilum was introduced in an environment exceeding pH 8.0, no growth was observed. Also, when the pH was lowered to 5.5 or exceeded 9.0, rapid cell lysis occured. Different salt living conditions were also observed for growth and life. Optimal salt conditionns were observed at 1.5% NaCl. Also, growth of cells were observed when there were no traces of NaCl in the solution; however, once NaCl concentrations exceeded 3.6%, no growth was observed.
Pyrobaculum aerophilum was isolated using a BS medium, using the following (per liter of double distilled H20): NaHCO3, 2.2g; NH4Cl, 0.25g; KH2PO4, 0.07g; (NH4)2Fe(SO4)2 x 6H20, 2mg; (NH4)2Ni(SO4)2 x 2H2O, 2mg; NaSeO4, 0.1mg; Na2WO4 x 2H2O, 0.1mg; synthetic sea water, 125ml; trace mineral solution, 10ml; marine medium, 125ml. Salt concentrations of the medium were altered using NaCl. The pH of the medium was adjusted using H2S04. Oxygen was reduced by adding 0.05% Na2S x 9H2O witht resazurin as the redox indicator. The original samples obtained from Maronti Beach, Italy had conditions ranging from 97 to 102°C and pH values from 5.5 to 6.5, respectively. The samples were transferred to serum tubes, which were then tightly stoppered.
Describe the appearance, habitat, etc. of the organism, and why it is important enough to have its genome sequenced. Describe how and where it was isolated.
Include a picture or two (with sources) if you can find them.
Genome structure
Pyrobaculum aerophilum is composed of a 2.2-megabase genome sequence. A whole genome analysis was computerized and experiments have confirmed that Pyrobaculum aerophilum and possibly all Crenarchaea lack 5’ untranslated regions in their mRNAs, and therefore do not appear to use a ribosome binding site. The lengths and dispersion of mononucleotide repeat-tracts uncovered that the Gs and Cs are highly precarious. This suggests that the archaeum could be lacking in a mismatch repair system.
Complete Genome Sequence, completed 12/12/2001 @ UCLA/CalTech
Summary: Length: 2,222,430 nt
GC Content: 51%
% Coding: 88%
Topology: circular
Molecule: dsDNA
Genes: 2706
Protein coding: 2605
Structural RNAs: 96
Pseudo genes: none
Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence?
Does it have any plasmids? Are they important to the organism's lifestyle?
Cell structure and metabolism
Pyrobaculum aerophilum has characteristics of both Thermoproteus and Pyrobaculum. The cylindrical rod-shape, the formation of terminal spheres, and the S-layer shows a close relationship to Thermoproteus and Pyrobaculum. However, the archaeum was unable to grow by sulfur respiration and growth was suppressed by the presence of elemental sulfur. The arcaheum was also found to grow by dissimilatory nitrate reduction, organic compounds, and thiosulfate. Interestingly, nitrate reduction has only been observed within the heterotrophic extreme halophiles.
Pyrobaculum aerophilum have a cell wall exhibiting a S-layer stationed on top of a cytoplasmic membrane. The S-layer was 5 nm in length on average and was anchored to the cytoplasmic membrane by a inner elongated domain (a spacer) that was 25 nm in length on average. The S-layer was consistent with other members of the genus Pyrobaculum including: P. islandicum, P. organotrophum, T.tenax.
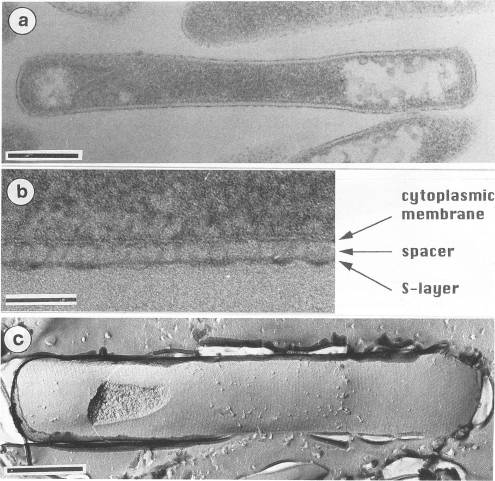
Describe any interesting features and/or cell structures; how it gains energy; what important molecules it produces.
Ecology
Describe any interactions with other organisms (included eukaryotes), contributions to the environment, effect on environment, etc.
Pathology
How does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
Application to Biotechnology
Does this organism produce any useful compounds or enzymes? What are they and how are they used?
Current Research
1. Discovery of a thermophilic protein complex stabilized by topologically interlinked chains.
"A growing number of organisms have been discovered inhabiting extreme environments, including temperatures in excess of 100 degrees C. How cellular proteins from such organisms retain their native folds under extreme conditions is still not fully understood. Recent computational and structural studies have identified disulfide bonding as an important mechanism for stabilizing intracellular proteins in certain thermophilic microbes." In this study, "the first proteomic analysis of intracellular disulfide bonding in the hyperthermophilic archaeon Pyrobaculum aerophilum is presented. The study reveals that "the utilization of disulfide bonds extends beyond individual proteins to include many protein-protein complexes. The 1.6 A crystal structure of one such complex, a citrate synthase homodimer. The structure contains two intramolecular disulfide bonds, one per subunit, which result in the cyclization of each protein chain in such a way that the two chains are topologically interlinked, rendering them inseparable. This unusual feature emphasizes the variety and sophistication of the molecular mechanisms that can be achieved by evolution."
Background: "The Archaea are highly diverse in terms of their physiology, metabolism and ecology. Presently, very few molecular characteristics are known that are uniquely shared by either all archaea or the different main groups within archaea. The evolutionary relationships among different groups within the Euryarchaeota branch are also not clearly understood."
Results: "Comprehensive analyses on each open reading frame (ORFs) in the genomes of 11 archaea (3 Crenarchaeota--Aeropyrum pernix, Pyrobaculum aerophilum and Sulfolobus acidocaldarius; 8 Euryarchaeota--Pyrococcus abyssi, Methanococcus maripaludis, Methanopyrus kandleri, Methanococcoides burtonii, Halobacterium sp. NCR-1, Haloquadratum walsbyi, Thermoplasma acidophilum and Picrophilus torridus) have been carried out to search for proteins that are unique to either all Archaea or for its main subgroups. These studies have identified 1448 proteins or ORFs that are distinctive characteristics of Archaea and its various subgroups and whose homologues are not found in other organisms. Six of these proteins are unique to all Archaea, 10 others are only missing in Nanoarchaeum equitans and a large number of other proteins are specific for various main groups within the Archaea (e.g. Crenarchaeota, Euryarchaeota, Sulfolobales and Desulfurococcales, Halobacteriales, Thermococci, Thermoplasmata, all methanogenic archaea or particular groups of methanogens). Of particular importance is the observation that 31 proteins are uniquely present in virtually all methanogens (including M. kandleri) and 10 additional proteins are only found in different methanogens as well as A. fulgidus. In contrast, no protein was exclusively shared by various methanogen and any of the Halobacteriales or Thermoplasmatales. These results strongly indicate that all methanogenic archaea form a monophyletic group exclusive of other archaea and that this lineage likely evolved from Archaeoglobus. In addition, 15 proteins that are uniquely shared by M. kandleri and Methanobacteriales suggest a close evolutionary relationship between them. In contrast to the phylogenomics studies, a monophyletic grouping of archaea is not supported by phylogenetic analyses based on protein sequences."
Conclusion: "The identified archaea-specific proteins provide novel molecular markers or signature proteins that are distinctive characteristics of Archaea and all of its major subgroups. The species distributions of these proteins provide novel insights into the evolutionary relationships among different groups within Archaea, particularly regarding the origin of methanogenesis. Most of these proteins are of unknown function and further studies should lead to discovery of novel biochemical and physiological characteristics that are unique to either all archaea or its different subgroups."
3. CC1, a novel crenarchaeal DNA binding protein.
"The genomes of the related crenarchaea Pyrobaculum aerophilum and Thermoproteus tenax lack any obvious gene encoding a single-stranded DNA binding protein (SSB). SSBs are essential for DNA replication, recombination, and repair and are found in all other genomes across the three domains of life. These two archaeal genomes also have only one identifiable gene encoding a chromatin protein (the Alba protein), while most other archaea have at least two different abundant chromatin proteins. We performed a biochemical screen for novel nucleic acid binding proteins present in cell extracts of T. tenax. An assay for proteins capable of binding to a single-stranded DNA oligonucleotide resulted in identification of three proteins. The first protein, Alba, has been shown previously to bind single-stranded DNA as well as duplex DNA. The two other proteins, which we designated CC1 (for crenarchaeal chromatin protein 1), are very closely related to one another, and homologs are restricted to the P. aerophilum and Aeropyrum pernix genomes. CC1 is a 6-kDa, monomeric, basic protein that is expressed at a high level in T. tenax. This protein binds single- and double-stranded DNAs with similar affinities. These properties are consistent with a role for CC1 as a crenarchaeal chromatin protein."
Enter summaries of the most recent research here--at least three required
References
Edited by student of Rachel Larsen and Kit Pogliano.
