Rhodotorula glutinis
Classification
Higher order taxa
Domain: Eukaryota
Phylum: Fungi
Class: Microbotryomycetes
Order: Sporidiobolales
Family: Sporidiobolaceae
Genus: Rhodotorula
Species
Rhodotorula glutinis
|
NCBI: [1] |
Description and significance
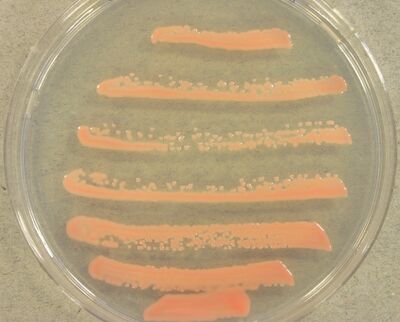
Rhodotorula glutinis, also known as “red yeast” (1), is a species of fungus in the Sporidiobolaceae family that is commonly found in the environment and on human skin (2). It has pink and red colonies due to its ability to produce red-orange carotenoid pigments (3). The species has been the focus of attention in the medical field, as it can persist on plastic surfaces, such as catheters, and potentially cause sepsis in immunocompromised patients (4). Current research focuses on the industrial significance of R. glutinis, including its ability to produce carotenoids, act as a biocatalyst, and aid in biofuel production (3, 5-6). Research suggests that R. glutinis can sustainably produce biofuel precursors from industrial and organic waste, but questions still remain about the cost-effectiveness of large-scale biofuel production utilizing R. glutinis (7, 8).
Genome structure
The Rhodotorula glutinis genome has 20,478,880 nucleotides, and guanine and cytosine (G + C) bases compose 61.9% of the genome. The region of the genome thought to encode genes contains 17,229,291 base pair and there are 3,359 genes coding for 2,817 proteins (9). Some of the major protein groups encoded in the genome play roles in the metabolism of lipids and carotenoids, along with other genes that produce enzymes that are highly relevant to the bio-products and pharmaceutical industries. One example of this is the enzyme phenylalanine ammonia lyase, which can be used to treat phenylketonuria, a disease that is caused by the build-up of the amino acid phenylalanine (10).
Morphology, Cell Structure, and Reproduction
Rhodotorula glutinis forms pink or red, smooth, moist, and mucus-like colonies when grown on agar. When fully matured, R. glutinis cells form an elongated sphere, or ellipsoid, and their diameter ranges from three to five micrometers (11). The overall structure and division of the nucleus is similar to other previously reported fungi and yeast. In some strains of R. glutinis, vacuoles are absent in young cells, suggesting they are not permanent structures and instead hold a transient function in the cell. Granular and rod-shaped mitochondria are often present in the cytoplasm (12). The nucleus consists of a central nucleolus surrounded by highly condensed DNA, and is enclosed by a nuclear membrane.
R. glutinis reproduces by simultaneously budding from multiple start points, also known as multipolar budding. Through this method, a smaller version of the yeast cell grows from the original cell. Eventually, the mature daughter cell separates from the mother cell, taking half of the nucleus and resulting in two identical cells (13). R. glutinis can also reproduce sexually through the use of mycelial clamp connections, which form a link between two fungal cells and transfers a nucleus, and with it DNA and genetic material, from one yeast cell to another (13).
Metabolic processes
Rhodotorula glutinis, like most fungi, is a chemoorganoheterotroph. R. glutinis generates energy through the breakdown of organic molecules with a preference for free monosaccharides and the disaccharides sucrose, trehalose, maltose, cellobiose, and lactose (14). Glucose has been identified as an optimal carbon source for R. glutinis, allowing for rapid growth and high carotenoid production (15). R. glutinis, unlike other well-studied species of yeast, is an aerobic organism that lacks the capacity to perform sugar fermentation (16). Typical of the overwhelming majority of eukaryotic organisms, R. glutinis utilizes the organic molecules NADH and FADH2 as electron carriers and molecular oxygen as a final electron acceptor (17).
Ecology
Rhodotorula glutinis is able to survive in a large variety of environments. In nature, it can be found in the air, soil, ocean water, and on the surfaces of leaves, and it has the potential to colonize humans and other mammals (18). It can also be found in refrigerated food products, particularly dairy (cheese, yogurt, butter), meats, and vegetables (18). Its optimal growth temperature is 28℃, although it can grow at temperatures both below it (refrigerator, ~4℃) and above it (human body, ~37℃), albeit not as quickly. Its optimal pH is 5.5 - 7.5 (19).
Pathology
Rhodotorula glutinis is a common component of the human and indoor microbiome, having been isolated from the air, skin, nails, soil, shower curtains, and toothbrushes (20). It also appears to survive well on plastic surfaces and is common in hospitals, with the Rhodotorula genus being the most commonly found yeast genus on the hands of medical workers (2, 21). The genus is resistant to several common antifungal medications, such as fluconazole and caspofungin (22). These qualities make R. glutinis — and the genus as a whole — a concern for immunocompromised patients and those with medical devices that remain in the body, such as catheters or vascular access devices. There have been many documented cases of death and serious illness following R. glutinis infection, such as catheter-related sepsis, keratitis (inflammation of the cornea), meningitis, and fungemia after solid-organ transplantation (23, 24, 25, 26). Of these illnesses, fungemia, or presence of fungal cells in the blood, is most common and causes symptoms such as fever, chills, and malaise (27, 28).
Industrial Applications
The benefits of Rhodotorula glutinis in various industrial and environmental applications are still being investigated, yet early findings are essential to our understanding of the fungus as well as its potential usage. R. glutinis can use monosodium glutamate (MSG) wastewater as a culture medium, in which it produces an optimal amount of lipids for biofuel use without needing as much oxygen when the proper amount of glucose is present (29). This demonstrates the utility of this fungus as a biofuel producer while simultaneously decreasing [ https://en.wikipedia.org/wiki/Pollutant pollutants] that reduce free oxygen for other organisms to use in the environment through waste runoff. This study also raises questions about what other wastewater products can be used as media to grow R. glutinis, which allows for further reduction of waste.
R. glutinis can also act as a biopesticide for blackberries, as it inhibits the growth of the infectious fungus Botrytis cinerea, which causes disease in the plant. Strains of R. glutinis isolated from blackberries were applied to blackberries at the same time as B. cinerea, leading to a decrease in disease incidence as R. glutinis inhibited the growth of B. cinerea (30).
Current Research
Current research of Rhodotorula glutinis is primarily focused on the production of biofuels and carotenoids. One study investigated the use of R. glutinis as a producer of biofuel using sugar beet molasses as a substrate in non-sterile conditions. Optimal lipid production occurred in the range of temperatures between 20 and 35° C, resulting in a much greater percentage of 16- and 18-carbon length fatty acid chains] (7). Such 16- and 18-carbon fatty acid chains are key for biofuel production, and its production throughout such a wide range of temperatures makes it easier for production in an industrial setting. Recent studies have also investigated the improvement of carotenoid production through the transformation of R. glutinis with cellulase genes and additional β-carotene production genes. These engineered strains can use organic cellulose material to amplify β-carotene production, introducing a potential application of R. glutinis in the efficient and sustainable breakdown of organic waste (31). Carotenoids are also utilized as antioxidants and are converted into vitamin A by the human body, making them a potential target for the pharmaceutical industry (32).
Rhodotorula glutinis is also shown to produce biofuel in the presence of both industrial and organic waste. One study regarding the use of R. glutinis in a recycling capacity showed that it can produce biofuels and biogas from fruit and vegetable waste. This type of organic waste is generally difficult to break down, yet, the fungus can grow in this material. In doing so, it produces a variety of fatty lipids from waste under the optimal conditions by first breaking it down with heat (6). This study shows the conversion of waste into energy, this time in the form of organic material versus industrial waste. More recent studies have shown significant biofuel production from a newly isolated strain of R. glutinis, T13. Biofuel produced from R. glutinis T13 has a concentration of fatty acids with properties similar to diesel, which makes it a potential competitor (33). More research is needed in order to make waste-treatment and fungus growth cost effective and optimal for biofuel production via R. glutinis.
References
1. Kot, A.M., S Błażejak, A Kurcz, et al. 2017. Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electronic Journal of Biotechnology, 27: 25-31.
2. Wirth, F. and L.Z. Goldani. 2012. Epidemiology of Rhodotorula: an emerging pathogen. Interdisciplinary Perspectives on Infectious Diseases. 2012: 465717.
3. Li, C., C. Zhenming, L. Jing, W. Xianghong. 2007. Enhanced carotenoid production by a mutant of the marine yeast Rhodotorula sp. Hidai. Journal of Ocean University of China 6: 66-71.
4. Miceli, M. H., J. A. Diaz, and S. A. Lee. 2011. Emerging opportunistic yeast infections. Lancet 11(2):142-151.
5. Kurbanoglu E. B., K. Zibeyaz, M. Ozdal, M. Taskin, N. I. Kurbanoglu. 2010. Asymmetric reduction of substituted acetophenones using once immobilized Rhodotorulus glutinis cells. Bioresource Technology. 101(11):3835-3829.
6. Razaghi, A., O. P. Karthikeyanab, H. T. Nguyen Hao, and K. Heimannan. 2016. Hydrolysis treatments of fruit and vegetable waste for production of biofuel precursors. Bioresource Technology 217: 100-103.
7. Taskin, M, S. Ortucu, M. N Aydogan, N. P Arslan. 2016. Lipid production from sugar beet molasses under non-aseptic culture conditions using the oleaginous yeast Rhodotorula glutinis TR29. Renewable Energy. 66: 198-204.
8. Razaghi, A., O. P. Karthikeyanab, H. T. Nguyen Hao, and K. Heimannan. 2016. Hydrolysis treatments of fruit and vegetable waste for production of biofuel precursors. Bioresource Technology 217: 100-103.
9. Paul D., Z. Magbanua, M. Arick, T. Ffrench, S. Bridges, S. Burgess, M. Lawrence. 2014. Genome Sequence of the Oleaginous Yeast Rhodotorula glutinis ATCC 204091. Genome Announcements 2(1): e00046-14.
10. Li, CJ., D Zhao, P. Cheng, et al. 2020. Genomics and lipidomics analysis of the biotechnologically important oleaginous red yeast Rhodotorula glutinis ZHK provides new insights into its lipid and carotenoid metabolism. BMC Genomics. 21(834).
11. Hernández-Almanza, A. J.C. Montanez, M.A. Aguilar-González, C Martínez-Ávila, R Rodríguez-Herrera, C.N. Aguilar. 2014. Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Bioscience 5: 64-72.
12. Thyagarajan T.R., H.B. Naylor. 1961. Cytology of Rhodotorula Glutinis. Journal of Bacteriology 83(1): 127-136.
13. Banno, I. 1967. Studies on Sexuality of Rhodotorula. Journal of General and Applied Microbiology. 13: 167-196.
14. Janda, S., Hedenström, M.V. 1974. Uptake of disaccharides by the aerobic yeast Rhodotorula glutinis. Archives of Microbiology. 101, 273–280.
15. Nam, H.S., J.S. Rhee. 1991. Effect of Carbon Source and Carbon to Nitrogen Ratio on Carotogenesis of Rhodotrula glutinis. Journal of Microbiology and Biotechnology. 1(1): 75-78
16. Kot, A.M., S. Błażejak, A. Kurcz, I. Gientka, M. Kieliszek. 2016. Rhodotorula glutinis - potential source of lipids, carotenoids, and enzymes for use in industries. Applied Microbiology and Biotechnology. 100: 6103-6117.
17. Lorenz, E., D. Runge, A.M. Marbà-Ardébol, M. Schmacht, U. Stahl, M. Senz. 2017. Systematic development of a two-stage fed-batch process of lipid accumulation in Rhodotorula glutinis. Journal of Biotechnology. 248: 4-15
18. Dworecka-Kaszak, B, M. Kizerwetter-Świda. 2011. Pseudomycelium forming Rhodotorula – unusual picture of biofilm. Mikologia Lekarska 18(2):74-78.
19. Zhao, Y, L. Guo, Y. Xia, X. Zhuang, W. Chu. 2019. Isolation, Identification of Carotenoid-Producing Rhodotorula sp. from Marine Environment and Optimization for Carotenoid Production. Marine Drugs. 17(3): 161.
20. Pfaller M.A. and D.J. Diekema. 2004. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. Journal of Clinical Microbiology. 42(10): 4419-31.
21. Strausbaugh L.J., D.L. Sewell, R.C. Tjoelker, T. Heitzman, T. Webster, T.T. Ward, and M.A. Pfaller. 1996. Comparison of three methods for recovery of yeasts from hands of health-care workers. Journal of Clinical Microbiology. 34(2): 471-3
22. Diekema D.J., B. Petroelje, S.A. Messer, R.J. Hollis, and M.A. Pfaller. 2005. Activities of available and investigational antifungal agents against Rhodotorula species. Journal of Clinical Microbiology. 43(1): 476-478.
23. Hsueh P.R., L.J. Teng, S.W. Ho, and K.T. Luh. 2003. Catheter-related sepsis due to Rhodotorula glutinis. Journal of Clinical Microbiology. 41(2): 857-9.
24. Guerra R., G.M. Cavallini, L. Longanesi, C. Casolari, G. Bertoli, F. Rivasi, and U. Fabio. 1992. Rhodotorula glutinis keratitis. International Journal of Ophthalmology. 16(3): 187-90.
25. Lanzafame M., G. De Checchi, A. Parinello, and M.T.A.M Cattelan. 2001. Rhodotorula glutinis-related meningitis. Journal of Clinical Microbiology, 39(1): 410-410.
26. Riedel D. J., J. K. Johnson, and G. N. Forrest. 2007. Rhodotorulus glutinis fungemia in a liver-kidney transplant patient. Transplant Infectious Disease. 10(3): 197-200.
27. Tuon F.F. and S.F. Costa. 2008. Rhodotorula infection. A systematic review of 128 cases from literature. Rev Iberoam Micol. 25(3):135-40.
28. Lo Re, V,, N.O. Fishman, and I. Nachamkin. 2003. Recurrent catheter-related Rhodotorula rubra infection. Clinical Microbiology and Infection, 9(8): 897-900.
29. Xue F., J. Miao, X. Zhang, H. Luo, T. Tan. 2008. Studies on lipid production by Rhodotorula glutinis fermentation using monosodium glutamate wastewater as culture medium. Bioresource Technology 99(13): 5923-5927.
30. Zapata J., C. Acosta, A. Diaz, L.Villamizar, A. M. Cotes. 2011. Characterization of Rhodotorula glutinis and Pichia onychis isolates with potential as biopesticides for Controlling Botrytis cinerea. Acta Horticulturae 905(905): 155-160.
31. Pi H.W., M. Anandharaj, Y.Y. Kao, Y.J. Lin, J.J. Chang, W.H. Li. 2018. Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous β-carotene and cellulase production. 2018. Scientific Reports 8(1): 10850.
32. Bhosale, P., R.V. Gadre. 2001. Optimization of carotenoid production from hyper-producing Rhodotorula glutinis mutant 32 by a factorial approach. Letters in Applied Microbiology 33(1): 12-16.
33. Maza D.D., S.C. Viñarta, E. García-Ríos, J.M. Guillamón, M.J. 2021. Aybar. Rhodotorula glutinis T13 as a potential source of microbial lipids for biodiesel generation. J Biotechnol. 331: 14-18.
Authorship Statement
AK- Classification, Introduction, Pathology, Editing, Wiki Upload
AS- Morphology, Cell Structure, Reproduction, Ecology, Editing
DA- Reproduction, Ecology, Editing
JF- Early Findings, Current Research, Editing
WD- Metabolic Processes, Current Research, Editing
Edited by students of Jennifer Talbot for BI 311 General Microbiology, 2021, Boston University.
