Rickettsia conorii: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
[[Image:JCS01382F1.jpg|frame|none|Preincubation of Vero cells with cytochalasin D diminished the ability of R. conorii to invade. Bar, 2 µm.] | [[Image:JCS01382F1.jpg|frame|none|Preincubation of Vero cells with cytochalasin D diminished the ability of R. conorii to invade. Bar, 2 µm.]] | ||
| Line 37: | Line 37: | ||
''Rickettsiae'' just like other bacteria enter into non-phagocytic host cells and adhere to it's vacuole(Hackstadt, 1996). Within the cytoplasm, ''rickettsiae'' begin to divide and able to polymerize host actin filaments to drive themselves to be linked intra- and intercellularly (Gouin et al., 2004; Gouin et al., 1999; Heinzen et al., 1993; Teysseire et al., 1992). | ''Rickettsiae'' just like other bacteria enter into non-phagocytic host cells and adhere to it's vacuole(Hackstadt, 1996). Within the cytoplasm, ''rickettsiae'' begin to divide and able to polymerize host actin filaments to drive themselves to be linked intra- and intercellularly (Gouin et al., 2004; Gouin et al., 1999; Heinzen et al., 1993; Teysseire et al., 1992). | ||
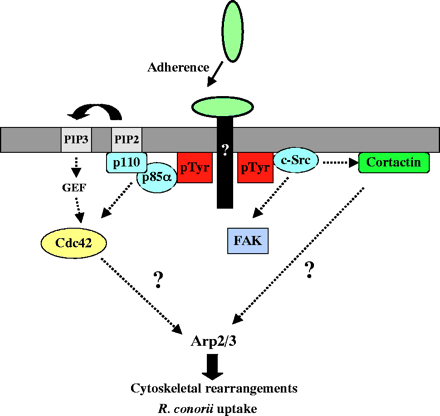
To internalize rickettsiae is associated with a phospholipase A2 activity and host actin polymerization (Silverman et al., 1992; Walker et al., 2001; Walker, 1984). R. conorii invades non-phagocytic cells. Some proteins are able to control actin dynamics during R. conorii invasion reveals that the Arp2/3 complex is recruited to the entry site. R. conorii uses pathways involving Cdc42, PI 3-kinase, c-Src and other PTK activities to enter non-phagocytic cells and that signals from these pathways may be coordinated to activate the Arp2/3 complex. Various signaling pathways by activating one signal to either supress or futher activate the Arp2/3 complex . Internalization of R. conorii most likely involves R. conorii surface protein(s) with an unidentified host cell receptor(s) [[Image:JCS01382F8.gif|frame|none] | To internalize rickettsiae is associated with a phospholipase A2 activity and host actin polymerization (Silverman et al., 1992; Walker et al., 2001; Walker, 1984). R. conorii invades non-phagocytic cells. Some proteins are able to control actin dynamics during R. conorii invasion reveals that the Arp2/3 complex is recruited to the entry site. R. conorii uses pathways involving Cdc42, PI 3-kinase, c-Src and other PTK activities to enter non-phagocytic cells and that signals from these pathways may be coordinated to activate the Arp2/3 complex. Various signaling pathways by activating one signal to either supress or futher activate the Arp2/3 complex . Internalization of R. conorii most likely involves R. conorii surface protein(s) with an unidentified host cell receptor(s) [[Image:JCS01382F8.gif|frame|none]] | ||
This picture shows the interactions involved in the uptake of Rickettsia conorii in non-phagocytic mammalian cells. | This picture shows the interactions involved in the uptake of Rickettsia conorii in non-phagocytic mammalian cells. | ||
Revision as of 19:17, 7 June 2007
A Microbial Biorealm page on the genus Rickettsia conorii
Classification
Higher order taxa:
Bacteria ; Proteobacteria ; Alphaproteobacteria ; Rickettsiales ; Rickettsiaceae ; Rickettsieae ; Rickettsia ; spotted fever group ; Rickettsia conorii
Gene Classification based on COG functional categories
Species:
Orientia tsutsugamushi;
spotted fever group: Candidatus R. principis; Israeli tick typhus rickettsia; R. aeschimannii; R. africae; R. akari; R. amblyommii; R. andeana; R. australis; R. conorii; R. cooleyi; R. felis; R. heilongjiangensis; R. heilongjiangii; R. helvtica; R. honei; R. hulinensis; R. hulinii; R. japonica; R. martinet; R. massiliae; R. monacensis; R. montanensis; R. moreli; R. parkeri; R. peacockii; R. rhipicephali; R. rickettsii; R. sibirica subgroup; R. slovaca; R. sp.
Typhus group: R. canadensis; R. prowazekii; R. typhi
Unclassified Rickettsia: Candidatus R. tarasevichiae; R. bellii; R. publicis; R. sp.
|
NCBI: Taxonomy Genome: -R. conorii str. Malish 7 -R. prowazekii str. Madrid E |
Description and Significance
Rickettsia bacteria are a pathogen transmitted to humans by Rhipicephalus ticks.[1] Rickettsia conorii are known as a cause of "Mediterranean spotted fever, Astrakhan fever, Israeli spotted fever, and Indian tick typhus in the Mediterranean basin and Africa, Southern Russia, Middle East, and India, respectively."[1] Israeli spotted fever and Mediterranean spotted fever overlap among clinical features and are characterized by a rash. The differences lies at an inoculation eschar,Indochina scrub typhus, is rarely in Astrakhan fever and Israeli spotted fever but common in Mediterranean spotted fever and is contracted by contact with infected brown dog ticks.
Genome Structure
The genome of Rickettsia conorii is 1,268,755 base pairs in length and contains 1374 protein-coding genes.(2) In anaerobic glycolysis, there are no genes in the biosynthesis and regulation of amino acids and no genes in the nucleosides of free-living bacteria. Rickettsia are intracellular small gram-negative proteobacteria in a subdivision associated with different arthropod hosts. The genomes and the mitochondria of Rickettsia are small, highly derived, "products of several types of reductive evolution" (Andersson et al. 1998). The R. conorii genome sequence relates closely with its relative Rickettsia prowazekii shows a new type of "coding" mobile element (Rickettsia-specific palindromic element, RPE)mostly found inserted in frame within open reading frames (ORFs). All bacterial palindromic repeats appeared exclusively in noncoding regions. There are 656 interspersed repeated sequences in 10 distinct families. Among the 10 families, three palindromic sequence families showed clear cases of insertions into open reading frames (ORFs). The in-frame insertion located to be compatible with three dimentional encoded protein.
Cell Structure and Metabolism
Rickettsiae just like other bacteria enter into non-phagocytic host cells and adhere to it's vacuole(Hackstadt, 1996). Within the cytoplasm, rickettsiae begin to divide and able to polymerize host actin filaments to drive themselves to be linked intra- and intercellularly (Gouin et al., 2004; Gouin et al., 1999; Heinzen et al., 1993; Teysseire et al., 1992).
To internalize rickettsiae is associated with a phospholipase A2 activity and host actin polymerization (Silverman et al., 1992; Walker et al., 2001; Walker, 1984). R. conorii invades non-phagocytic cells. Some proteins are able to control actin dynamics during R. conorii invasion reveals that the Arp2/3 complex is recruited to the entry site. R. conorii uses pathways involving Cdc42, PI 3-kinase, c-Src and other PTK activities to enter non-phagocytic cells and that signals from these pathways may be coordinated to activate the Arp2/3 complex. Various signaling pathways by activating one signal to either supress or futher activate the Arp2/3 complex . Internalization of R. conorii most likely involves R. conorii surface protein(s) with an unidentified host cell receptor(s)
This picture shows the interactions involved in the uptake of Rickettsia conorii in non-phagocytic mammalian cells.
Ecology
R. conorii is a boutonneus fever disease and it is spread in mediteeranean countries. It is divided into two groups, the spotted fever group and the typhus group(Vishwanath, 1991). It's mostly carried by ticks or fleas on animals. This disease are distributed in a lot of different areas around the world. If it is infected with this disease, it will result with a dermal rash (Hand et al., 1970; Walker et al., 1988). Bacteria can spread via lymphatic vessels to the lymph nodes by bloodstream to other tissues like the lungs, spleen, liver, etc(Walker and Gear, 1985).
Pathology
My organism R. conorii is caused by boutonneuse fever. It came from an African origin. Eliza tests to examine specimens, the results show in the early stage of a high percentage of the symptoms and it lasted a long time after the acute attack. Spotted fever group of rickettsiae are maintained in nature through transovarial transmission. In the tick, it is very critical that ovaries are transmitted of rickettsiae from one generation to the next. Ticks can usually maintain several species of rickettsiae, but only a single species is tranferred transovarilly. Phages are found over 100 in bacterial gnera and also rickettsiae.
Below are tables of different groups of Ricketsia along with the diseases that each species cause and their general geological distribution. From The University of South Carolina.
Spotted Fever Group
| Organism | Disease | Distribution |
| R. rickettsii | Rocky Mountain spotted fever | Western hemisphere |
| R. akari | Rickettsialpox | USA, former Soviet Union |
| R. conorii | Boutonneuse fever | Mediterranean countries, Africa, India, Southwest Asia |
| R. sibirica | Siberian tick typhus | Siberia, Mongolia, nothern China |
| R. australis | Australian tick typhus | Australia |
| R. japonica | Oriental spotted fever | Japan |
Typhus Group
| Organism | Disease | Distribution |
| R. prowazekii |
Epidemic typhus |
South America and Africa Worldwide United States |
| R. typhi | Murine typhus | Worldwide |
Scrub typhus group
| Organism | Disease | Distribution |
| R. tsutsugamushi | Scrub typhus | Asia, northern Australia, Pacific Islands |
Application to Biotechnology
Does this organism produce any useful compounds or enzymes? What are they and how are they used? Rickettsia conorii revealed under microsope some pathologic features and presence of rickettsiae in the endothelium of infected tissues. Some key antioxidant enzymes involved "namely glutathione peroxidase, glutathione reductase, glucose-6-phosphate dehydrogenase, and superoxide dismutase, at these times exhibited a pattern of differential and selective modulation in brain, lungs, and testes of mice infected with viable organisms, whereas heat-inactivated or sonically disrupted rickettsiae had no effect" [4 under current research in reference]. The involvent of these enzymes of glutathione redox and superoxide scavenging systems in the antioxidant response depends on the viable rickettsiae in different organs of the host.
Current Research
1)A case of acute quadriplegia complicating Mediterranean spotted fever
Rickettsia conorii caused mediterranean spotted fever and this was consider to be a benign disease. However, about 10% of the patients with severe symptom are neurologic involved. A case of a 80 year old man was studied with R. conorii infection. A characterisitic of tache noire was diagnosed on the lateral region of the thigh. After running a immunofluorescence test, elevated IgM antibody was detected against R conorii and it was talked about it in this research paper.
2)Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain
This research paper talks about tests from cats for IgG antibodies to "Rickettsia conorii (Rc), Ehrlichia canis (Ec), Anaplasma phagocytophilum (Ap) and Bartonella henselae (Bh) antigens using IFA and for FeLV antigen and FIV antibody by ELISA"[2 under current research column of reference]. PCR testing was performed and Bh antibodies were detected with seroreactivity to both Ec and Rc antigens and FIV antibodies were involve with illness and cats older than 2 years.
3)Characterisation of rickettsial diseases in a hospital-based population in Malta
The aim of the paper was to find out the causative agents of rickettsial disease in Malta and epidemiology of cases related to the disease. Thirty-three cases studied. One patient was diagnosed of Rickettsia conorii but none of the sera showed any activity with Rickettsia typhi. Spotted fever rickettsiosis is endemic in Malta. None of the cases were due to murine typhus. The causative agent of rickettsial disease in Malta should be R. conorii,
References
General:
- Yong Zhu, Pierre-Edouard Fournier, Marina Eremeeva, and Didier Raoult1. 2004. "Proposal to create subspecies of Rickettsia conorii based on multi-locus sequence typing and an emended description of Rickettsia conorii." Biomed Central.
- Reinert, Birgit. 2001. "Insights into genome evolution: The sequence of Rickettsia conorii." Genome News Network
- Chantal Abergel, Guillaume Blanc, Vincent Monchois, Patricia Renesto, Cécile Sigoillot, Hiroyuki Ogata, Didier Raoult and Jean-Michel Claverie. 2006. "Impact of the Excision of an Ancient Repeat Insertion on Rickettsia conorii Guanylate Kinase Activity ." Oxford Journals.
- Andersson, Siv G. E., Alireza Zomorodipour, Jan O. Andersson, Thomas Sicheritz-Ponten, U. Cecilia M. Alsmark, Raf M. Podowski, A. Kristina Naslund, Ann-Sofie Eriksson, Herbert H. Winkler, and Charles G. Kurland. 1998. "The genome sequence of Rickettsia prowazekii and the origin of mitochondria." Nature, vol. 396. Macmillan Publishers Ltd. (133-143)
- Walker, D. H. and Gear, J. H. (1985). Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am. J. Trop. Med. Hyg. 34, 361-371.[Medline]
Cell Structure and Metabolism
- Juan J. Martinez and Pascale Cossart. 2004. "Early signaling events involved in the entry of Rickettsia conorii into mammalian cells."Journal of Cell Science 117, 5097-5106
- Silverman, D. J., Santucci, L. A., Meyers, N. and Sekeyova, Z. (1992). Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect. Immun. 60, 2733-2740.[Abstract/Free Full Text]
- Hackstadt, T. (1996). The biology of rickettsiae. Infect. Agents Dis. 5, 127-143.[Medline]
- Gouin, E., Egile, C., Dehoux, P., Villiers, V., Adams, J., Gertler, F., Li, R. and Cossart, P. (2004). The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427, 457-461.[CrossRef][Medline]
current research
- Santo Caroleo, Chiara Longo, Domenico Pirritano, Rita Nisticò, Paola Valentino, Maurizio Iocco, Ermenegildo Santangelo and Bruno Amantea. 2007. A case of acute quadriplegia complicating Mediterranean spotted fever. Clinical Neurology and Neurosurgery.
- Laia Solano-Gallego, Barbara Hegarty, Yvonne Espada, Joan Llull and Edward Breitschwerdt. 2006.
Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Veterinary Microbiology.
- I. Tonna, C. Mallia Azzopardi, T. Piscopo, P. Cuschieri, F. Fenollar and D. Raoult. 2006. Characterisation of rickettsial diseases in a hospital-based population in Malta. Journal of Infection.
- Elena Rydkinaa, Sanjeev K. Sahni, , a, Lisa A. Santuccib, Loel C. Turpina, Raymond B. Baggsc and David J. Silvermanb. 2003. Selective modulation of antioxidant enzyme activities in host tissues during Rickettsia conorii infection. University of Maryland School of Medicine.
Edited by Cindy Zhang,student of Rachel Larsen and Kit Pogliano