Secondary Metabolites in the Aspergillus Genus
Introduction
By Helen Rogers
Aspergillus, a genus comprised of filamentous fungi, is a widely studied group of sac fungi containing a diverse array of both beneficial and pathogenic species. Like all fungi, Aspergillus species are eukaryotic and have a cell wall made of chitin. They are heterotrophic; most commonly found in soil habitats, but are also found in marine environments. Some common species include Aspergillus fumigates, responsible for the highest number of human deaths from fungi, "Aspergillus flavus", a destructive agricultural pest, and Aspergillus nidulans, an important model organism for (Gibbons and Rokas 2013). A wide variation in metabolism, reproductive strategies, and survival mechanisms are found among Aspergillus members, rendering them not only as resilient and diverse microbes, but also as important organisms for biological study.
All Aspergillus produce asci (sexual spore producing cells) within ascocarps (circular fruiting bodies). Some members have the ability to reproduce both sexually and asexually, depending on favorable or unfavorable environmental conditions. This reproductive strategy adds to their resilience and commonality in a diverse range of ecosystems.
Many "Aspergillus" species also possess the ability to form symbiotic relationships with other fungi, bacteria, algae, plants, and even insects. That said, many species are also pathogenic, and many species account for a majority of plant and human pathogens.
A defining characteristic of "Aspergillus" is their ability to produce secondary metabolites in response to environmental parameters, allowing them to adapt to complex and changing environments. These secondary metabolites allow fungi to either increase their own fitness or decrease a surrounding organism’s fitness, ensuring survival and reproduction. Despite significant scholarship surrounding the "Aspergillus" genus, their production of secondary metabolites remains a relatively unexplored area of study.
The metabolic and reproductive characteristics of Aspergillus, as outlined above, render this genus important both in microbial research and in other biological topics, such as genetics, bioengineering, ecology, and biochemistry.
Aspergillus Metabolism
Primary metabolism of Aspergillus relies on a distinct set of fatty acid synthase complexes that build fatty acid chains from the breakdown of carboxylic acids. During fungal growth and development, primary metabolism generates long fatty acid chains, which are used for cell membrane production and the formation of vesical storage bodies. In contrast, secondary metabolism largely involves polyketide synthases that generate polyketides molecules from distinct acidic compounds. For example, hexanoyl coA is used as a building block for the secondary metabolite sterigmatocystin which has both immunosuppressant abilities and carcinogenic qualities. Distinct synthase complexes used for the biosynthesis of different metabolic compounds ensure complex and precise regulation of both metabolisms, often, it is the case that the activation of secondary metabolism is associated with the inhibition of primary metabolic pathways (Brown et. al. 1996).
Secondary Metabolites
The ability for filamentous fungi to produce secondary metabolites from altered or de novo biosynthetic pathways remains a topic of scientific interest. Both the degree of secondary metabolism use and the diversity of secondary metabolites found in Aspergillus still largely remain unknown. This is largely a result of secondary biosynthetic pathways that remain silent or inactive under normal laboratory conditions (Nutzmann et. al. 2011). Therefore, it is difficult to study the mechanisms and signaling pathways behind secondary metabolism activation. Nevertheless, metabolites produced are molecules essential to pharmaceutical, industrial, and agricultural interests and applications.
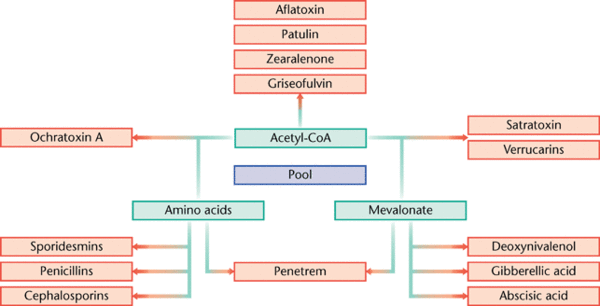
Secondary metabolites are structurally diverse low molecular mass molecules that are not essential for the growth and survival of the producing organism. Differing from the essential role of primary metabolites, secondary metabolites instead serve to increase the fitness of the producing organism or to decrease the fitness of surrounding organisms (Brakhage 2013). The major groups of secondary metabolites found in Aspergillus include polyketides (PKs), ribosomal and nonribosomal peptides (NRPs) and terpenoids (Anderson et. al. 2013). The diverse nature of secondary metabolites is a result of many tailoring enzymes that infer biochemical modifications on an otherwise standard structural polymer (Anderson et. al. 2013). As a result, a complex and intricate level of genetic regulation exists. The genes that code for secondary metabolism are organized in clusters, which encode for primary enzymes, and are flanked by genes that code for regulatory, transporter, or signaling proteins (Brakhage 2013).
A few of the many metabolites synthesized by Aspergillus that have been discovered hold great importance in human application; a few examples include the cholesterol reducing drug lovastin, the antibiotic penicillin, and the pathogenic human toxin aflatoxin (Gibbons and Rokas 2013).
Regulation of Secondary Metabolites
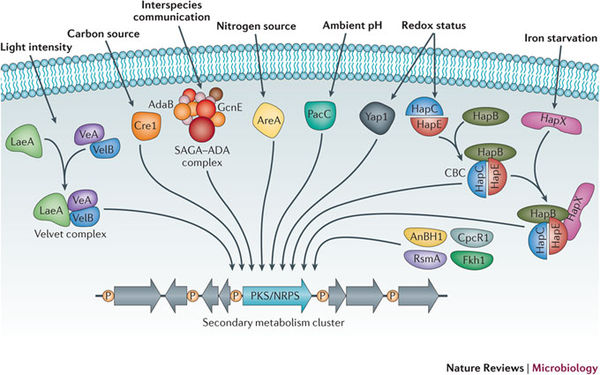
The regulation of secondary metabolism and their subsequent synthesis pathways is controlled by posttranslational modification (Nutzmann et. al. 2011). Studies have shown that histone modification by acetyltransferase complexes of regulatory genes or transcription factors leads to the induction of secondary metabolite production. Similar to DNA methylation, chromatin is reprogrammed which leads to the increased transcription of secondary metabolite gene clusters (Nutzmann et. al. 2011). This complex regulatory network is responsive to various environmental stimuli, including but not limited too, carbon and nitrogen levels, temperature, light, pH, and stimuli from other organisms (Brakhage 2013). These transcription factors are responsive to these stimuli, and when activated, these factors act on the chromatin scaffold comprised of histone proteins, that DNA then wraps around. Phosphorylation, methylation, and acetylation, are all various modifications that are associated with the regulation of secondary metabolism gene clusters (Brakhage 2013). The bacteria, streptomycete, cause an interesting biological example of secondary metabolic induction via mediated histone acetylation. Nutzmann et. al. (2011) outlined the fact that actinomycete bacterial species can induce histone modification in Aspergillus nidulans via the main histone acetytransferase (HAT) complex. It was found that the physical interaction of fungal mycelium and Streptomyces microbes activated the secondary metabolic pathway that resulted in the production of the polyketide orsellinic acid. This metabolic pathway was induced by the activation of the ors gene cluster and the saga/ada complex. Studies such as these illustrate the sensitivity of secondary metabolite production in relation to the surrounding environment, and demonstrate that physical stimuli as well as chemical signals can regulate SM production (Nutzmann et. al. 2011). Likewise, it demonstrates that many complex and relatively unknown ecological factors are involved in the activation of transcription factors inducing secondary metabolite activity.
However diverse the regulation of SM production remains, recent study has shown that a nuclear protein, LaeA is a key master regulatory protein in most Aspergilla species. It has been shown that the knockout of this gene results in widespread reduction of most SM levels. VeA protein has also been identified as a key regulatory protein, increasing expression levels in response to light stimuli. VeA and LaeA interact together to regulate SM production, asexual development, and sporulation. Upregulation of these genes has been found to directly increase secondary metabolite molecules such as sterigmatocystin, terrequinone, and aflatoxins (Fox and Howlett 2008). As demonstrated, the knockout of key genes is an important tool for further elucidation of SM regulation by proteins (transcription factors).
The Relationship Between Sporulation and Secondary Metabolite Synthesis
Calvo et. al. (2002) laid the groundwork for the association between fungal development and secondary metabolite levels. Calvo et. al. (2002) found that most secondary metabolites are produced after the fungus has gone through its initial growth and development, and is entering a stage of development characterized by spore formation. This resting stage is coupled with the onset of asexual sporulation and the rise of secondary metabolites. Differing from normal vegetative growth, the resting stage is initiated by certain environmental conditions and is controlled by g-protein growth pathways. Thus, the rise of secondary metabolite synthesis in conjunction with asexual sporulation, leads to the hypothesis that metabolites play a role in initiation, regulation, and process of sporulation is Aspergillus species (Calvo et. al. 2002).
The reproductive process of the model organism, Aspergillus nidulans best illustrates the relationship between secondary metabolite production and sporulation, and its advantageous fitness benefits. A. nidulans, and other Aspergillus species, have the ability to produce both sexual and asexual spores. Sexual ascospores are produced inside cleisththecia fruiting bodies in favorable environmental conditions. However, A. nidulans are also able to produce conidia (asexual spores) in specialized structures called conidiophores during conditions favoring conservatism and survival. Conidiophore formation begins with a stalk that begins extending from a thick walled foot cell. The stalk then begins to invert, forming a vesicle, and specialized cells called sterigmata result. From sterigmata, conidial spores are released into the environment and dispersed. Conidial reproduction is regulated by gene, brlA, which activates condial developmental genes resulting in asexual spore production (Calvo et. al 2002).
It has been established, that secondary metabolites play an integral role in sporulation activity. Metabolites can act as important sporogenic factors, acting as a key trigger for asexual spore production. For example, the SM sterigmatocystin in A. nidulans has been shown to increase asexual spore production, inhibit the production of primary metabolite kinases, and increase hyphal branching. Other secondary metabolites also function as fungal pigments, or melanins, found within conidiophores. Fungal melanins are dark pigments deposited in the cell wall manufactured by a secondary biosynthesis pathway that oxidizes phenols. Melanin compounds are important because they increase the virulence of many fungi. For instance, high melanin levels promote the formation of appressoria, specialized cell structures that increase fungal infection rate by promoting penetration and entrance into a host plant. How melanin levels affect the virulence of human pathogenic fungi remains to be determined.
Secondary metabolites can not only increase the success and rate of sporulation in the individuals that produce these compounds, but can also decrease competition in its surrounding environment, in order to ensure successful spore germination. Mycotoxins, a secondary metabolite group of potent and carcinogenic toxins, manufactured and secreted by species such as Aspergillus parasticus and Aspergillus nidulans are an effective poison to many competing plant, animal, and microbial species in the surrounding environment. The expression of all of these secondary metabolites that in turn influence the onset of asexual sporulation are dependent on environmental conditions such as temperature, nutrient availability, and pH. However, the interaction between environmental conditions and secondary metabolite signaling pathways that lead to an increase in sporulation activity remains to be studied (Calvo et. al. 2002).
Human Applications of Aspergillus and Effects of Secondary Metabolites
As mentioned above, the use of "Aspergillus" secondary metabolites can be seen in several human medical applications such as the antibiotic penicillin (Gibbons and Rokas 2013). These human applications largely represent the use of recombinant genetics technologies and other bioengineering techniques. Other human applications of such metabolites include commercial food applications such as the use of "Aspergillus oryzae", a close relative of the filamentous fungi, "Penicillium paxilli", in the production of sake, soy sauce, and other fermented goods (Rank et. al. 2009).
According to Rank et. al. (2009), some concern over the use of "A. oryzae" in food applications arose when the similarities between "A. oryzae" and "A. flavus" secondary metabolites were discovered. It was found that "A. flavus" displayed roughly 99.5% genetic homology in relation to "A. oryzae" and 98.0% homology at the protein level. However, contrasting from the innocuous "A. oryzae", "A. flavus" is efficient in the production of a recognized mycotoxin, a carcinogenic compound known as the aflatoxin. It is estimated that the two species, "A. oryzae and A. flavus", share nearly the same number of genes and thus express very similar coding for the synthesis of very distinct secondary metabolites. How homologous genes and nearly identical biosynthesis pathways produce contrasting metabolites, one toxic to humans and one useful for fermentation of foods still needs to be elucidated. Numerous laboratory studies conducted comparing the chemistry of "A. flavus" and "A. oryzae" have found no aflatoxin synthesis on the part of A. oryzae in isolated environments. That said, several other documented mammalian pathogens produced by "A. flavus" populations have also been found present in "A. oryzae" populations. These toxins found in both species include cyclopiazonic and kojic acids. Only the aflatoxins, seen as the toxin set to pose the greatest threat, have been fully documented in both species, however, much of the other secondary metabolites of these specific Aspergillus species remain largely unknown and potentially undiscovered.
Larger examples of the human application of "Aspergillus include the commercial production of chymosin, a rennin found in rennet and an enzyme critical to the production of cheese, which was traditionally derived from the stomachs of ruminants. Chymosin derived from A. niger was the first genetically-modified food additive to be used on a commercial scale (Vogt et. al. 2001). Additionally, "Aspergillus members", specifically "A. niger" individuals are heavily used in the commercial process for citric acid production and are responsible for producing nearly one billion pounds of citric acid annually, using the Embden-Myerhof pathway. Both the production of chymosin and citric acid are examples of human use of "Aspergillus" where relatively little is known about these secondary metabolite pathways being utilized for human benefit. Not unlike most of the Aspergillus species, because none of the documented secondary metabolites of "A. niger" are known to pose a significant pathogenic threat (such as the threat posed by aflatoxins), research and sequencing of this particular species has been fairly limited (Vogt et. al. 2001).
An Example of Posttranscriptional Change: Carbon Starvation
A common stress for Aspergillus species is the limiting availability of carbon, eliciting the carbon starvation stress response (CSSR). As a result of limited carbon uptake, a drastic alteration is undergone in many metabolic and biosynthetic pathways. CSSR is akin to starvation, because without a carbon source, fungi no longer have a steady energy source required for normal growth and development. The CSSR involves a number of metabolic and molecular changes within the fungi, including programmed cell death, an increase in secondary metabolite production, and a rise in digestive enzyme levels. The degradation of the fungal cell wall by hydrolytic enzymes is important for the liberation of nutrients and energy storing molecules found within the cell wall. An increase in the expression of genes encoding for stress proteins was also found. Biosynthesis pathways that involve the regulation of glycolysis from the breakdown of glucose are down regulated in favor of degradation of carbohydrates, lipids, and nitrogenous molecules freed from the breakdown of the cell wall (Szilagyi et. al. 2012).
Coupled with these changes, the production of secondary metabolites increases. Such metabolites include mycotoxins, antibiotic and antimycotic molecules, and fungal pigments. Szilagyi et. al. (2012) explored the role of regulatory transcription factors involved in CSSR. It was found that the BrlA gene, encoding for conidial development and thus asexual sporulation, was also involved in the formation of stress response proteins under carbon starvation conditions. In addition, the alcR gene was found to play an important role in eliciting CSSR metabolic changes. The regulatory AldA proteins break down acetaldehyde for energy; a rise in acetaldehyde levels is a result of the initial degradation of amino acids. The aflrR gene was also upregulated, increasing the aflatoxin regulatory protein, AflR. AflR is then though to trigger the production of sterigmatocystin, which has antibacterial and antifungal properties. The carbon starvation response in fungi illustrates the complexity of secondary metabolite regulation. As described, many secondary metabolites have multiple functions with the cell. In addition, SM production is upregulated or dowregulated by a multiple of transcriptional factors and post-transcriptional modifications. However, all of these processes come together in order to ensure fungal survival (Szilagyi et. al 2012).
Conclusion
The biosynthesis of secondary metabolites is often underestimated, and thus, largely understudied in the genus Aspergillus. Aspergillus are a genus comprised filamentous fungi which display a diverse set of metabolic pathways in order to survive complex environmental conditions. Nonessential secondary metabolites synthesized in response to environmental triggers allow Aspergillus species to maintain a distinct fitness advantage in a competitive environment. Indeed, these metabolites are of great interest above and beyond the microbial realm, including importance in medicinal, industrial, and agricultural fields. For example, it has been shown that the majority of anti-carcinogenic and tumor suppressing molecules utilized in modern medicine have origin in natural metabolites (Calvo et. al. 2012).
Important pharmaceutical drugs, including penicillin and lovastatin are metabolites synthesized by fungi. Bioengineering of more resistant and resilient crop strains by the incorporation of fungal metabolites has already taken place. Many industrial chemicals, and their enzymatic formation, also originate from naturally synthesized metabolites (Brakhage 2013). Metabolites synthesized by Aspergillum are also important in the efficiency of dairy fermentation, the manufacturing of sake, and play a role in food spoilage (Calvo et. al. 2002). Contrastingly, metabolites such as mycotoxins, enable widespread fungal virulence in both plants and animals and have been documented as a leading cause of crop destruction and human diseases such as aspergillosis (Brakhage 2013).
Future research should focus on upregulation of gene coding for beneficial secondary metabolites, knocking out the synthesis of harmful toxic metabolites, and better understanding of the environmental conditions that trigger the activation and regulatory factors of secondary metabolic pathways. Due to the widespread silence of secondary metabolite genes under normal laboratory conditions and the multitude of factors influencing posttranscriptional regulation of metabolic pathways, valuable study of secondary metabolites is still needed (Fox and Howlett 2008). A better understanding of histone modification, chromatin reprogramming, and epigenetic influences should be examined. Thus, secondary metabolites remain as an important topic of study for genetics, cell biology, ecology, and molecular biology. The fungal genus, Aspergillum, provide important model species that enable the study of these secondary metabolites (Anderson et. al. 2013).
References
Brakhage, Axel A. "Regulation of fungal secondary metabolism." Nature Reviews Microbiology (2012).
Demain, Arnold L. "Microbial biotechnology." Trends in Biotechnology 18, no. 1 (2000): 26-31
[http://www.researchgate.net/publication/47634067_Aspergillus_nidulans_asexual_development_making_the_most_of_cellular_modules?ev=pub_cit Etxebeste, Oier, Aitor Garzia, Eduardo A. Espeso, and Unai Ugalde. "Aspergillus nidulans asexual development: making the most of cellular modules." Trends in microbiology 18, no. 12 (2010): 569-576.]
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2013, Kenyon College.