Sediment Electrogens
Sediment Electrogens
Introduction
Certain guilds of microbes have a physiological ability to generate electric charge by oxidizing organic substances and donating electrons, to metal ions that serve as an anode (Hong, et al. 2009). The general term for these organisms is electrogens, or electrigens.
Physical environment
Electrogens can be found in many different environments. Common examples include: freshwater sediments, marine sediments, groundwater, andwastewater. Sediment environments are favorable, as oxygen must be removed as the terminal electron acceptor in order for electrogens to produce exogenous charge (Logan & Regan). Composition of sediments is determined by their origin. As marine sediments only refer to a collection of immiscible solids present in an aqueous environment, physical properties of marine sediments are highly variable. Three categories of sediments can be formed based on their origins, and composition.

Lithogenous Sediments
Lithogenous sediments are products of rock deformation, and consist of small, inorganic particles formed by ionic bonds. These usually take the form of metal silicates.
Biogenous Sediments

Biogenous sediments, also termed biogenic ooze, are compacted remains of prior living tissue, calcified bone, and plankton (Aiello & Bekins, 2010). These sediments often accumulate as hydroxylapatite, a primary component of scale and bone.
Hydrogenous Sediments
Hydrogenous sediments originate from reactions of water with existing sediment. They are often found in concentrated clusters or “nodules” of manganese and iron oxides.
Microbial communities
Two of the most commonly observed genera are Geobacteria and Shewanella – both members of the Proteobacteria phylum. A more recently studied genus, Geothrix is a member of the Acidobacteria phylum, and is different in metabolic processes compared to Geobacteria and Shewanella.
Geobacteraceae
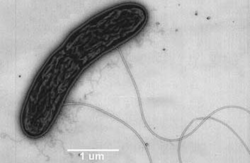
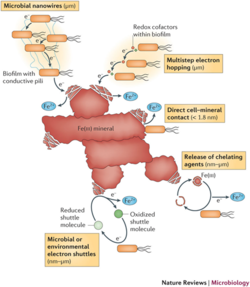
Geobacteracae are members of the phylum Proteobacteria. Bacteria of the Geobacteracae family are prevalent in anoxic marine sediments, as they are strictly anaerobic (Bond & Lovley, 2003). Species from this family are able to use alternate electron acceptors, commonly Iron (III) oxide in the species G. sulfurreducens (Leang, Coppi, & Lovley, 2003). Biofilms of G. sulfurreducens are able to grow proximally to each other. Daughter cells grow on top of mother cells, producing layers of cells that interchange membrane potentials amongst all members of the biofilm colony (Marsili, et al. 2008). This is accomplished via multiple membrane transport proteins (Bond & Lovley, 2005).
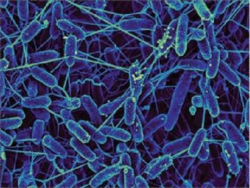
Shewanellaceae
Shewanellaceae are also members of the phylum Proteobacteria. They are a diverse group of bacteria, capable of thriving in solid and aqueous environments - both freshwater and saltwater (Sikora et al., 2011). Shewanella oneidensis is able to reduce magnesium as a terminal electron acceptor, by the action of an extracellular cytochrome c protein (Myers & Myers, 1997). This cytochrome allows for reduction of not only insoluble metals, but nitrate and sulfate as well. While other electron acceptors are common, Shewanella are facultative anaerobes, and can in some cases deposit electrons onto molecular oxygen (D R Lovley, 1991).
Geothrix fermentans
Geothrix are members of the phylum Acidobacteria. They are also able to use metals as terminal electron acceptors. Familiar organic compounds, such as acetate serve as electron donors, while metallic ions (typically iron) serves as an electron acceptor (Bond & Lovley, 2005). Geothrix are the least versatile of bacteria found in marine sediments, as they cannot as easily deposit extracellular charge as Geobacter spp. and Shewanellaceae spp. .
Microbial processes
Microbial success in marine sediments is dependent upon their ability to perform anaerobic respiration or fermentation due to the limited availability of oxygen as a terminal electron acceptor. From this step, two different mechanisms can deliver the charge extracellularly via proteins. Type IV pili, found in Geobacter spp. and cytochrome containing outer membrane extensions found in Shewanella spp ( Lovley & Malvankar, 2015) (Pirbadian et al., 2014). Other species are also capable of transferring electrons through cycling between intermembrane spaces and the exterior sedimentary environment (Harris et al., 2010), or discharged onto electron transporters through diffusible transport proteins (Rabaey, et al. 2005).
Iron Cycling
Microbial dissimilatory iron reduction (DIR) is an active biogeochemical process in marine sediments (Crosby, Roden, Johnson, & Beard, 2007). Two different forms of iron are generally present in marine sedimentary environments. Fe3+ is insoluble, and accumulates on sediment granules, while Fe2+ is a soluble cation, making it more readily available for extracellular uptake. Ferrous Iron (III) can be oxidized or reduced, coupled with negatively charged humic products as electron donors, utilizing the Iron as an electron acceptor. Geobacter are capable of performing complete reduction of Iron (III) to magnetite (Fe3O4) with different electron donors – utilization of monoaromatics, short chain fatty acids, and hydrogen has been observed (Melton, Swanner, Behrens, Schmidt, & Kappler, 2014).
Carbon Cycling
Electrogens are able to completely oxidize organic metabolites to carbon dioxide. Increases of dissolved carbon dioxide outside of marine sediments can prove beneficial for marine algal and plant life. The ability of dissimilatory iron-reducing bacteria to break down organic matter trapped in or beneath sediments could result in a trickle-up effect, making use of otherwise hidden organic compounds and making them bioavailable for species at higher water levels (Azam & Fenchel, 1983).
Current Research
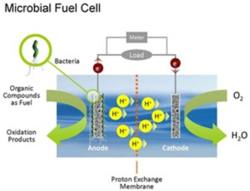
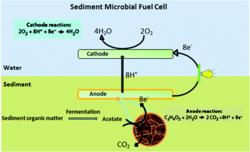
Researchers are observing successes of electrogens in engineering of microbial fuel cells. Fuel cells can be divided into two subcategories: mediator- required microbial fuel cells, and mediator-independent microbial fuel cells. Mediator- required microbial fuel cells require an intermediate to carry the charge from redox reactions to ions or anodes. Mediator-independent microbial fuel cells can transfer charge to metal ions or anodes. Mediator- required MFC’s are not as practical, as they require use of electron acceptor molecules that are typically toxic and expensive to purchase industrially (Li, 2010). Mediator-independent fuel cells utilize electrogenic bacteria to produce an extracellular charge. Input chemicals can be derived from wastewater, sediments, or even plant life (Strik, 2008).
Microbial fuel cells are a two-fold method of reducing environmental contaminants. They allow for cleaning of aqueous organic contaminants (wastewater for instance), and electricity production without fossil fuel byproducts. An additional use of MFC’s is remediation of accumulated organic matter in marine environments. Too much organic matter can impede marine organisms by decreasing available oxygen and increasing dissolved methane (Campbell, Twiss, & Wilkinson, 2011). Fuel cells could be dually helpful in current environmental issues by biodegrading contaminants in marine environments, harnessing energy derived from contaminant metabolism, as well as increasing bioavailability of nutrients for other marine organisms.
References
Aiello, I. W., & Bekins, B. A. (2010). Milankovitch-scale correlations between deeply buried microbial populations and biogenic ooze lithology. Geology, 38(1), 79–82. http://doi.org/10.1130/G30207.1
Azam, F., Fenchel, T., Field, J., Gray, J., Meyer-Reil, L. & Thingstad, F. (1983) The Ecological Role of Water-Column Microbes in the Sea. Marine Ecology Progress Series, 10, 257–263
Bond, D. R., & Lovley, D. R. (2003). Electricity production by Geobacter sulfurreducens attached to electrodes. Applied and Environmental Microbiology, 69(3), 1548–55. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=150094&tool=pmcentrez&rendertype=abstract
Bond, D. R., & Lovley, D. R. (2005). Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Applied and Environmental Microbiology, 71(4), 2186–9. http://doi.org/10.1128/AEM.71.4.2186-2189.2005
Campbell, P. G., Twiss, M. R., & Wilkinson, K. J. (2011). Accumulation of natural organic matter on the surfaces of living cells: implications for the interaction of toxic solutes with aquatic biota. Canadian Journal of Fisheries and Aquatic Sciences. Retrieved from http://www.nrcresearchpress.com/doi/abs/10.1139/f97-161#.VvnsAZRdVu4
CROSBY, H. A., RODEN, E. E., JOHNSON, C. M., & BEARD, B. L. (2007). The mechanisms of iron isotope fractionation produced during dissimilatory Fe(III) reduction by Shewanella putrefaciens and Geobacter sulfurreducens. Geobiology, 5(2), 169–189. http://doi.org/10.1111/j.1472-4669.2007.00103.x
Harris, H. W., El-Naggar, M. Y., Bretschger, O., Ward, M. J., Romine, M. F., Obraztsova, A. Y., & Nealson, K. H. (2010). Electrokinesis is a microbial behavior that requires extracellular electron transport. Proceedings of the National Academy of Sciences of the United States of America, 107(1), 326–31. http://doi.org/10.1073/pnas.0907468107
Hong, S. W., Chang, I. S., Choi, Y. S., Kim, B. H., & Chung, T. H. (2009). Responses from freshwater sediment during electricity generation using microbial fuel cells. Bioprocess and Biosystems Engineering, 32(3), 389–395. http://doi.org/10.1007/s00449-008-0258-9
Leang, C., Coppi, M. V, & Lovley, D. R. (2003). OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. Journal of Bacteriology, 185(7), 2096–103. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=151516&tool=pmcentrez&rendertype=abstract
Li, J., Liu, G., Zhang, R., Luo, Y., Zhang, C., & Li, M. (2010). Electricity generation by two types of microbial fuel cells using nitrobenzene as the anodic or cathodic reactants. Bioresource Technology, 101(11), 4013-4020. doi:10.1016/j.biortech.2009.12.135
Logan, B. E., & Regan, J. M. (n.d.). Electricity-producing bacterial communities in microbial fuel cells. http://doi.org/10.1016/j.tim.2006.10.003 Lovley, D. R. (1991). Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Mol. Biol. Rev., 55(2), 259–287. Retrieved from http://mmbr.asm.org/content/55/2/259.short
Lovley, D. R., & Malvankar, N. S. (2015). Seeing is believing: novel imaging techniques help clarify microbial nanowire structure and function. Environmental Microbiology, 17(7), 2209–15. http://doi.org/10.1111/1462-2920.12708
Marsili, E., Rollefson, J. B., Baron, D. B., Hozalski, R. M., & Bond, D. R. (2008). Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Applied and Environmental Microbiology, 74(23), 7329–37. http://doi.org/10.1128/AEM.00177-08
Melton, E. D., Swanner, E. D., Behrens, S., Schmidt, C., & Kappler, A. (2014). The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nature Reviews. Microbiology, 12(12), 797–808. http://doi.org/10.1038/nrmicro3347
Myers, C. R., & Myers, J. M. (1997). Outer membrane cytochromes of Shewanella putrefaciens MR-1: spectral analysis, and purification of the 83-kDa c-type cytochrome. Biochimica et Biophysica Acta, 1326(2), 307–18. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9218561
Pirbadian, S., Barchinger, S. E., Leung, K. M., Byun, H. S., Jangir, Y., Bouhenni, R. A., … El-Naggar, M. Y. (2014). Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proceedings of the National Academy of Sciences of the United States of America, 111(35), 12883–8. http://doi.org/10.1073/pnas.1410551111
Rabaey, K., Boon, N., Höfte, M., & Verstraete, W. (2005). Microbial phenazine production enhances electron transfer in biofuel cells. Environmental Science & Technology, 39(9), 3401–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15926596
Schulz, H. D., & Zabel, M. (Eds.). (2006). Marine Geochemistry. Berlin/Heidelberg: Springer-Verlag. http://doi.org/10.1007/3-540-32144-6
Sikora, A., Wójtowicz-Sieńko, J., Piela, P., Zielenkiewicz, U., Tomczyk-Zak, K., Chojnacka, A., … Błaszczyk, M. (2011). Selection of bacteria capable of dissimilatory reduction of Fe(III) from a long-term continuous culture on molasses and their use in a microbial fuel cell. Journal of Microbiology and Biotechnology, 21(3), 305–16. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21464603
Strik, D. B., Hamelers (Bert), H. M., Snel, J. H., & Buisman, C. N. (2008). Green electricity production with living plants and bacteria in a fuel cell. International Journal Of Energy Research, 32(9), 870-876. doi:10.1002/er.1397
Zabihallahpoor, A., Rahimnejad, M., & Talebnia, F. (2015). Sediment microbial fuel cells as a new source of renewable and sustainable energy: present status and future prospects. RSC Adv., 5(114), 94171–94183. http://doi.org/10.1039/C5RA15279H
Edited by Adam Hall, a student of Mary Beth Leigh at the University of Alaska Fairbanks Template adapted from one used by Angela Kent at the University of Illinois at Urbana-Champaign.