Simian foamy virus: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Talbot2015}} | ||
The Simian Foamy Virus (SFV) is a spumavirus that primarily infects non-human primates [[#References|[1]]]. It is named after the foamy-looking syncytia that are formed from the fusion of neighboring cells following infection. SFV infects a significant number of primates bred in captivity which predisposes them to other viruses [[#References|[2]]]. This virus may have co-speciated with monkeys at least 30 million years ago, making it the oldest known vertebrate virus [[#References|[3]]]. Foamy viruses are the least well characterized of all [[Retroviridae|retroviruses]] [[#References|[4]]]. | The Simian Foamy Virus (SFV) is a spumavirus that primarily infects non-human primates [[#References|[1]]]. It is named after the foamy-looking syncytia that are formed from the fusion of neighboring cells following infection. SFV infects a significant number of primates bred in captivity which predisposes them to other viruses [[#References|[2]]]. This virus may have co-speciated with monkeys at least 30 million years ago, making it the oldest known vertebrate virus [[#References|[3]]]. Foamy viruses are the least well characterized of all [[Retroviridae|retroviruses]] [[#References|[4]]]. | ||
Latest revision as of 15:25, 12 February 2016
The Simian Foamy Virus (SFV) is a spumavirus that primarily infects non-human primates [1]. It is named after the foamy-looking syncytia that are formed from the fusion of neighboring cells following infection. SFV infects a significant number of primates bred in captivity which predisposes them to other viruses [2]. This virus may have co-speciated with monkeys at least 30 million years ago, making it the oldest known vertebrate virus [3]. Foamy viruses are the least well characterized of all retroviruses [4].
The virus is able to cross over into humans, although it is not currently known to be pathogenic in humans [5]. Any person who works with the bodily fluids of nonhuman primates in institutions such as animal sanctuaries, zoos, or medical research institutions is at risk for SFV; however, the long-term effects of this virus are unknown. Because SFV is a retrovirus, high levels of mutation and recombination are expected, making the spread of the virus harder to control should it mutate into a pathogenic strain. Moreover, since both SFV and HIV are retroviruses, SFV is a good model to study the effects and mechanisms of retroviral zoonosis, including the origin of Human Immunodeficiency Virus (HIV) [1].
Classification
Unasssigned (Order); Retroviridae (family); Spumaretrovirinae (subfamily); Spumavirus (Genus) Simian foamy virus (species) [6]
Genome structure
The simian foamy virus genome is made up of single-stranded sense RNA. Multiple strains of the simian foamy virus have been sequenced and catalogued, including those for old world primates and new world primates [1]. The simian foamy virus genome consists of five genes and two untranslated regions flanking these five genes. Of these genes, three (gag, pol, and env) are considered necessary and two (tas and bel2) are considered accessory. The gag gene encodes the viral capsid protein, the pol protein encodes the viral protease-reverse trasncriptase protein, and the env protein encodes the viral envelope protein, all of which are essential for viral function and formation. The tas gene encodes a transcriptional transactivator that binds to a region within the env gene to induce transcription of the tas gene [7]. The full sequences of SFV from the old world primates rhesus macaque (Macaca mulatta) [8] and African green monkey (Chlorocebus sabaeus) [9], from the new world primates spider monkey (Ateles ssp.) [10] and Brazilian capuchin monkey (Sapajus apella) [11], and from the apes Central chimpanzee (Pan troglodytes troglodytes) [12,13] and Western gorilla (Gorilla gorilla) [12] are available as well as others. The genomes of existing SFV strains are diverging at the slowest rate identified among retroviruses at 1.7 × 10-8 substitutions per site per year [3].
Viral structure
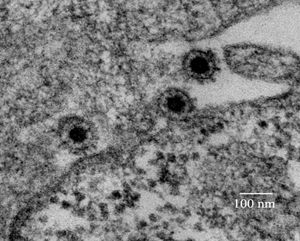
The viral particle (see image) has three major constituent proteins: the capsid protein encoded by the gag gene, the envelope protein encoded by the env gene, and the protease-reverse transcriptase protein encoded by the pol gene. Unlike other retroviruses, in order for the complete and mature viral capsid to form, the viral gag transcript is not cleaved into multiple structural polypeptides; rather, the SFV pol protease cleaves a larger capsid peptide into two smaller peptides, one of which becomes the major constituent of the viral capsid [14,15]. Without cleavage, SFV is non-infectious. The capsid protein necessarily interacts with the envelope protein and other capsid proteins in order to localize the capsid protein to the cellular envelope [16]; specifically, the Arg50 amino acid of the capsid protein is necessary for envelope protein association and thus viral budding [17]. The envelope protein is cleaved by human signal peptide peptidase-like 3 (SPPL3) and further cleavage is performed by additional SPP proteins [18], suggesting a potential mechanism against human pathogenicity. The pol protein functions as both a protease (used to cleave the gag peptide) and a reverse transcriptase [19]. It can switch between a regulated form (in which protease activity is restricted) and an active form in order to ensure correct gag cleavage.
Infection strategy
SFV does not have its own metabolic system, but it has a unique infection strategy. SFV is transmitted by an exchange of bodily fluids, mainly saliva. It is normally transmitted from primates to humans through bites [20].
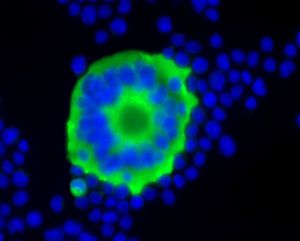
Although foamy viruses are retroviruses, they are complex viruses (as opposed to all other retroviruses, which are not complex) because they encode viral proteins that aren’t incorporated into viral particles [17]. Foamy viruses require the expression of an envelope protein that allows the intracellular capsids to bud [17]. This requires an interaction between Gag and Env proteins, and C-terminal Gag cleavage is essential for infectivity [17]. Unlike retroviruses, if the Gag protein in foamy viruses is lacking an N-terminus myristylation signal, capsid targeting to the plasma membrane is inhibited, even in the presence of the Env protein (which is necessary for particle aggregation and Gag cleavage) [17]. In foamy viruses, cytoplasmic targeting is involved with capsid assembly (see image). The Gag-Env interactions that drive particle budding may serve as a promising target for drug treatment of foamy viruses.
Although SFV itself is not pathogenic in humans, viral loads can become overwhelming with co-infection of multiple retroviruses. Primates infected with SFV and an additional virus have significantly higher viral loads of the second virus in their blood plasma, decreased CD4+T cells, and a higher number of deaths. SFVs also have a unique capsid assembly of foamy viruses [2]. There is an APOBEC3G-mediated hypermutation in the DNA of infected individuals [w1].
It is not clear how exactly simian foamy viruses infect humans. Also, despite equal exposure to the virus, the infection rates in humans vary [22]. There are multiple strains of SFV and many projects committed to discovering the exact mechanism of infection.
Ecology
Foamy viruses infect many species of mammals including primates, felines, bovines, and equines. Almost all wild, nonhuman primates are infected with SFV and include prosimians, baboons, macaques, monkeys, and apes. Each primate species has been shown carry a different strain of SFV (see image) [23]. The human version is referred to as human foamy virus (HFV), and it is significantly different from all simian foamy viruses.
The virus can be transferred from primates to humans via zoonosis. Zoonosis is the natural transmission of an infectious disease from an animal to a human. In the case of SFV, the zoonosis is direct meaning the virus can be transmitted directly through media like saliva or other bodily fluids [24].
Studies on SFV in natural settings in some central African countries including Cameroon and the Democratic Republic of the Congo illustrate effective transmission of the virus from nonhuman primates to humans, especially when following gorilla and ape bites during hunting incidents [25]. While direct zoonosis of SFV is common, there is no evidence of onward transmission from humans to other humans [21].
Pathology
Simian foamy viruses (SFVs) are found in nearly all wild nonhuman primates (NHP) in both active viral forms (found in oral mucosa) and latent proviral forms (found in a variety of cell types including blood). The suspected mechanism for infection is that latent proviral form is transferred between species via bites and infects Peripheral Blood Mononuclear Cells (PBMCs) which migrate to the oral mucosa and become pathogenic in NHPs after transcriptional activation [26]. The zoonotic transmission rate is lower in regions of the world with extended exposure to infected NHPs [24]. The mortality rate of SFV alone has not been quantified however studies suggest that rhesus macaques infected with both SFV and Simian Immunodeficiency Virus (SIV) had higher mortality rates [2]. Possible treatments for SFV may involve upregulation of the enzyme family APOBEC3G which is suspected to prevent viral replication by inducing hypermutations in the viral genome [22].
Current Research
SFV is important to study because it could potentially be used as a vector in gene therapy due to its lack of pathogenicity after zoonotic transfer to humans. Using smaller animals such as hamsters is beneficial in this research as they are less expensive and have more lenient regulations than required for nonhuman primate research.
There is a great deal of current research focused on SFV. First, a group of scientists found that in West Central Africa there is a large portion of the human population co-infected with HIV and SFV [27]. This suggests although SFV itself is not pathogenic, it aids the infection of other viruses. Additionally, SFV can be spread through fluids other than saliva [27]. The possibility of zoonosis to human has also led to a variety of research being performed regarding potential inhibitors. Recent research has identified that the antiviral protein complex APOBECG3 inhibits SFV replication in humans [26]. This may serve as a promising therapeutic target.
Researchers are also currently investigating populations in close, constant contact with multiple strains of SFV in Bangladesh. People residing in urban Bangladesh are consistently infected with multiple strains of SFV, several of which are recombinant [21]. Ongoing studies are investigating the animal acquisition and movement characteristics of nomadic human populations in Bangladesh in order to understand their resistance to infection. Isolating the source of SFV and how it mutates are important for treatment and infection prevention.
References
[1.] Rethwilm, A. (2010) Molecular biology of foamy viruses. Medical Microbiology and Immunology 199(3):197-207.
[2.] Choudhary, A., Galvin, T.A., Williams, D.K., Beren, J., Bryant, M.A., Khan, A.S. (2013) Influence of naturally occurring simian foamy viruses (SFVs) on SIV disease progression in the rhesus macaque (Macaca mulatta) model. Viruses 5(6):1414-30.
[3.] Switzer et. al. (2005) Ancient co-speciation of simian foamy viruses and primates. Nature 434: 376-380.
[4.] Meiering, C., Linial, M. (2001) Historical Perspective of Foamy Virus Epidemiology and Infection. Clinical Microbiology Reviews. 14(1):165-76
[5.] Wolfe, N.D., Switzer, W.M., Carr, J.K., Bhullar, V.B., Shanmugam, V., Tamoufe, U., Prosser, A.T., Torimiro, J.N., Wright, A., Mpoudi-Ngole, E., McCutchan, F.E., Birx, D.L., Folks, T.M., Burke, D.S. (2004) Naturally acquired simian retrovirus infections in central African hunters. Lancet 363(9413):932-7.
[6.] International Committee on Taxonomy of Viruses (July 2014). “Virus Taxonomy: 24th Release.” Retrieved November 1, 2015. << http://www.ictvonline.org/virusTaxonomy.asp?src=NCBI&ictv_id=20026748 >>
[7.] Campbell, M., Eng, C., Luciw, P.A. (1996) The simian foamy virus type 1 transcriptional transactivator (Tas) binds and activates an enhancer element in the gag gene. Journal of Virology 70(10):6847-55.
[8.] Kupiec, J.J., Kay, A., Hayat, M., Ravier, R., Péries, J., Galibert, F. (1991) Sequence analysis of the simian foamy virus type 1 genome. Gene 101(2):185-94.
[9.] Renne, R., Friedl, E., Schweizer, M., Fleps, U., Turek, R., Neumann-Haefelin, D. (1992) Genomic organization and expression of simian foamy virus type 3 (SFV-3). Virology 186(2):597-608.
[10.] Thumer, L., Rethwilm, A., Holmes, E.C., Bodem, J. (2007) The complete nucleotide sequence of a New World simian foamy virus. Virology 369(1):191-7.
[11.] Troncoso, L.L., Muniz, C.P., Siqueira, J.D., Curty, G., Schrago, C.G., Augusto, A., Fedullo, L., Soares, M.A., Santos, A.F. (2015) Characterization and comparative analysis of a simian foamy virus complete genome isolated from Brazilian capuchin monkeys. Virus Research 208:1-6.
[12.] Rua, R., Betsem, E., Calattini, S., Saib, A., Gessain, A. (2012) Genetic characterization of simian foamy viruses infecting humans. Journal of Virology 86(24):13550-9.
[13.] Herchenroder, O., Renne, R., Loncar, D., Cobb, E.K., Murthy, K.K., Schneider, J., Mergia, A., Luciw, P.A. Isolation, cloning, and sequencing of simian foamy viruses from chimpanzees (SFVcpz): high homology to human foamy virus (HFV). Virology 201(2):187-99.
[14.] Enssle, J., Fischer, N., Moebes, A., Mauer, B., Smola, U., Rethwilm, A. (1997) Carboxy-terminal cleavage of the human foamy virus Gag precursor molecule is an essential step in the viral life cycle. Journal of Virology 71(10):7312-7.
[15.] Zemba, M., Wilk, T., Rutten, T., Wagner, A., Flugel, R.M., Lochelt, M. (1998) The carboxy-terminal p3Gag domain of the human foamy virus Gag precursor is required for efficient virus infectivity. Virology 247(1):7-13.
[16.] Fischer, N., Heinkelein, M., Lindemann, D., Enssle, J., Baum, C., Werder, E., Zentgraf, H., Muller, J.G., Rethwilm, A. (1998) Foamy virus particle formation. Journal of Virology 72(2):1610-5.
[17.] Eastman, S.W., Linial, M.L. (2001) Identification of a conserved residue of foamy virus Gag required for intracellular capsid assembly. Journal of Virology 75(15):6857-64.
[18.] Voss, M., Fukumori, A., Kuhn, P.H., Kunzel, U., Klier, B., Grammer, G., Haug-Kroper, M., Kremmer, E., Lichtenthaler, S.F., Steiner, H., Schroder, B., Haass, C., Fluhrer, R. Foamy virus envelope protein is a substrate for signal peptide peptidase-like 3 (SPPL3). Journal of Biological Chemistry 287(52):43401-9.
[19.] Hartl, M.J., Wohrl, B.M., Rosch, P., Schweimer, K. (2008) The solution structure of the simian foamy virus protease reveals a monomeric protein. Journal of Molecular Biology 381(1):141-9.
[20.] Broussard, S., Comuzzie, G., Leighton, K., Leland, M., Whitehead, E., Allan, J. Characterization of New Simian Foamy Viruses from African Nonhuman Primates. Virology 237(2):349-59.
[21.] Engel G.A., Small C.T., Soliven K., Feeroz, M.M., Wang, X., Kamrul, Hasan, M., Oh, G., Rabiul Alam, S.M., Craig, K.L., Jackson, D.L., Matsen IV, F.A., Linial, M.L., Jones-Engel, L. (2013) Zoonotic simian foamy virus in Bangladesh reflects diverse patterns of transmission and co-infection. Emerging Microbes & Infections 2(9):58.
[22.] Soliven, K., Wang, X., Small, C.T., Feeroz, M.M., Lee, E.G., Craig, K.L., Hasan, K., Engel, G.A., Jones-Engel, L., Matsen, F.A., Linial, M.L. Simian foamy virus infection of rhesus macaques in Bangladesh: relationship of latent proviruses and transcriptionally active viruses. Journal of Virology 87(24):13628-39.
[23.] 1. Liu W., Worobey M., Li Y., Keele B.F., Bibollet-Ruche F., Guo Y., Goepfert, P.A., Santiago, M.L., Ndjango, J.B., Neel, C., Clifford, S.L., Sanz, C., Kamenya, S., Wilson, M.L., Pusey, A.E., Gross-Champ, N., Boesch, C., Smith, V., Zamma, K., Huffman, M.A., Mitani, J.C., Watts, D.P., Peeters, M., Shaw, G.M., Switzer, W.M., Sharp, P.M., Hahn, B.H. (2008) Molecular Ecology and Natural History of Simian Foamy Virus Infection in Wild-Living Chimpanzees. PLoS Pathogens 4(7):1000097.
[24.] Craig, K.L., Hasan, M.K., Jackson, D.L., Engel, G.A., Soliven, K., Feeroz, M.M., Wang, X., Jones-Engel, L., Linial, M.L. (2015) A seminomadic population in Bangladesh with extensive exposure to macaques does not exhibit high levels of zoonotic Simian Foamy Virus infection. Journal of Virology 89(14):7414-6.
[25.] Broussard, S., Comuzzie, G., Leighton, K., Leland, M., Whitehead, E., Allan, J. Characterization of New Simian Foamy Viruses from African Nonhuman Primates. Virology 237(2):349-59.
[26.] Matsen IV F.A., Small C.T., Soliven K., Engel G.A., Feeroz M.M., Wang X., Craig K.L., Hasan, M.K., Emerman M., Linial M.L., Jones-Engel, L. (2014) A novel bayesian method for detection of APOBEC3-mediated hypermutation and its application to zoonotic transmission of simian foamy viruses. PLoS Computational Biology 10(2):1003493.
[27.] Switzer, W.M., Garcia, A.D., Yang, C., Wright, A., Kalish, M.L., Folks, T.M., Heneine, W. (2008) Coinfection with HIV-1 and simian foamy virus in West Central Africans. Journal of Infectious Diseases 197(10):1389-93.
[28.] Calattini, S., Wanert, F., Thierry, B., Schmitt, C., Bassot, S., Saib, A., Herrenschmidt, N., Gessain, A. (2006) Modes of transmission and genetic diversity of foamy viruses in a Macaca tonkeana colony. Retrovirology 3:23.
Edited by Kevin Rhine, Sinead Nguyen, Deena Qadir, and Amber Thiel, students of Jennifer Talbot for BI 311 General Microbiology, 2015. Boston University
