Soil Environment
Introduction
The soil environment consists of a variety of physical, biological and chemical factors that affect the abundance and diversity of microbes found in the soil. [4] At its basic level, the soil environment consists of a solid and porous fraction. Within these fractions, a variety of chemical and physical factors are affected by and and affect microbes. These include, but are not limited to texture, temperature, pH, oxygen, cation exchange capacity and redox reactions.
The soil environment directly affects the types of microbes, as well as the rates of processes they perform. For example, microbial activity increases with temperature, which in turn affects rates of decomposition. On the other hand, microbial processes directly affect their environments as well, contributing to the carbon and nitrogen cycles, which are important for microbial and plant health. [5] At the micro scale, bacteria and other microbes participate in a variety of reactions that affect nutrient cycling, pH, as well as oxygen and CO2 content. At the macro scale, these processes can change the landscape in drastic ways, assisting in weathering of the soil and development of soil layers.
Soil Environment Overview
Solid Fraction
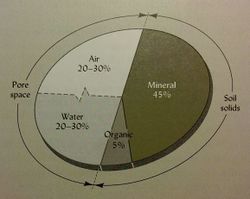
The solid fraction of the soil consists of mineral and organic matter, which is typically about 50% of the soil by volume (see figure 1), and it has a dominant influence on heat, water, and chemical transport and retention process. [4] Most of the solid particles are derived from mineral sources such as decomposed rocks or sediments. [13] Soil organic matter (SOM) consists of all of the organic components of a soil, including living biomass, decomposing tissue, and fully decomposed tissue called humus. [5] The rate and pathway of carbon decomposition and SOM formation directly affects the carbon cycle . Texture can also influence chemical properties such as cation exchange capacity (CEC). Finer textured soils with high clay content will have great CEC than soils with low clay content.
Soil Pores
Soil pores consist of the air and water filled fractions of the soil, and together they make up about 50% of the soil by volume. Pore space is largely determined by size and arrangement of aggregates and affects the movement of water, air, and organisms in soil. Soil pores are typically classified based on size: Macropores ( >75µm) Mesopores ( 30-70µm) Micropores ( 5-30µm) Ultramicropores (0.1-5µm) Cryptoporus ( <0.1µm). [5]
The air filled pores of the soil typically have a similar distribution of gases as the atmosphere above the soil, with slightly lower oxygen and slightly more CO2 due to the respiration of microorganisms. The soil atmosphere consists of about 18-20% oxygen near the surface, which decreases with depth. CO2 is around 1%, and N2 is about 78% of the soil air filled pore space. [5] Oxygen content will be lower when available carbon is high (demand for high O2 to utilize carbon). Soil that is high in clay content and/or compacted may have trouble exchanging gases to the atmosphere. Soil air generally has a very high moisture content when compared to the atmosphere (~100% unless the soil is very dry). The amount and composition of air in a soil is largely determined by the water content in the soil. [4]
The main source of soil water is rainfall and overland flow. The amount of water that enters the soil is a function of soil structure and texture. Water moves in soil through mass flow and capillary action. The water in soil is often called the soil solution, which can move nutrients from the surface through the soil column. The water fraction of the pores is typically between 20% to 30% but can vary depending on precipitation, soil texture, and soil structure. The water content of a soil influences gas exchange, nutrient movement and concentrations of nutrients, and buffers the temperature of the soil. [5]
Soil Aggregates & Structure
Soil aggregates are groups of soil particles that bind to each other more strongly than to adjacent particles. The space between the aggregates provide pore space for retention and exchange of air and water. Aggregation affects erosion, movement of water, and plant root growth. [7] Polysaccharides produced by soil bacteria, and humic substances and hyphae produced by fungi improve aggregation.
Soil structure is the arrangement of primary soil particles into aggregates which describes the arrangement of the solid parts of the soil and the pore spaces between them. Soil structure has a major influence on water movement, SOM leaching, and gas exchanges of the soils. The water movement, affected by structure can bring SOM in the surface to deep inside the soil. Soil with high clay content and/or poor structure, may have reduced infiltration and will cause runoff, erosion and surface crusting. [11] This can cause nutrient loss and increase the potential of desertification.
Physical & Chemical Factors that Control Biological Activity in the Soil
Texture
Soil texture is defined as the distribution of sand (0.05-2.0 mm), silt (0.002-0.05mm), and clay ( < 0.002mm) in soil. Soil texture indirectly influences properties such as: water holding capacity, porosity, aeration and nutrient availability. Clay particles have a very high surface to volume ratio, which makes them very chemically active and have high nutrient availability. Due to the adhesion of water, soils high in clay will also have a high water holding capacity. Soils with a high clay content will often have a very active microbial community, especially in areas the rhizosphere. [5]
One study by Hamarshid found that CO2 production rates were greatest in fine textured soil compared to coarse textured soil. [6] This was due to finer textured soils’ greater ability to hold nutrients and water, allowing microbial populations to thrive. However, this is not always the case, as other chemical or physical properties may affect the ability of microbes to perform processes such as carbon decomposition. (e.g. temperature, pH and quality of substrate).
Temperature
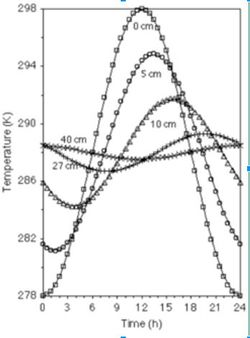
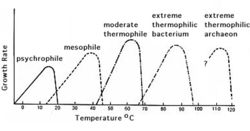
Soil temperature changes with depth: the surface soil (~0-20cm) is highly affected by the solar radiation. Moving deeper (~below 27cm) temperatures are very stable over time (see figure 5). This is because heat moves in soil mainly by conduction, which does not allow much heat to reach deep in the soil profile. Soil temperature is also affected by the soil color, soil cover, and the water content of the soil. A darker soil can absorb more heat compared to lighter color soil. A dry soil is more easily heated than a wet one due to the higher heat capacity of water. Heat moving in soil is analogous to the movement of water. [5] Generally, the higher the temperature, the more active microbes are, with microbial activity typically doubling with a 10° rise in temperature. However, some bacteria thrive at very low temperatures (psychrophiles) and very high temperatures (extremophiles), which can be seen in figure 6. [4]
pH
pH changes in soils is due to both biotic and abiotic processes. Microbes consume and release H+ through redox reactions and fermentation. Abiotic processes such as rainfall can also affect the pH of the soil. In areas of high rainfall, acidic soils can be created through leaching of bases from the soil, while more basic soils are typically located in arid environments. One study by Fierer et al. found that pH was the single most important factor influencing microbial diversity at the continental scale. [10] The study used 98 samples from across North and South America, and found that the greatest microbial diversity coincided with a neutral pH, even between two soil from the same forest.
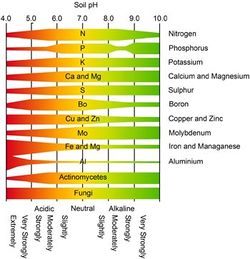
pH affects microbial diversity because many microbial species cannot tolerate extreme levels of pH (high or low). Alterations in pH can render essential microbe enzymes inactive and/or denature proteins within the cells and prevent microbial activity from occurring. [4] However, there are microbes that can withstand extreme pH environments. At pH below 5, fungi and acidophilic bacteria have a competitive advantage over other bacteria that thrive at a more neutral pH.
pH can also affect the availability of nutrients in this soil. Below a pH of 5, essential plant nutrients such as phosphorous, calcium and magnesium are not available. Low pH can also cause aluminum (Al3+) to be released from soil minerals. Al3+ in soil solution is not only toxic to plants and microbes, it can combine with OH- ions causing the free H+ ions to lower the pH further. [5] The effect of pH on nutrient availability and microbe survivability can be seen in figure 7.
Oxygen
Oxygen (O2) is a very important component of the productivity of both microbes and plant roots. Oxygen has a very high electrical potential (Eh), meaning that it has a lot of potential to produce energy when used as an electron acceptor in an oxidation-reduction reaction. [5] An example oxidation-reduction reaction can be seen in equation 1, where glucose is being oxidized, and oxygen is being reduced.
6O2+C6H12O6→6CO2+6H2O [eq.1]
The amount of oxygen available in a soil depends on a number of factors, including soil porosity, water content, and consumption by respiring organisms. If soil pores are large and interconnected, oxygen can flow easily. However, even in a well aerated soil, micro-aggregates may contain anaerobic zones in which oxygen flow is very limited. In a flooded soil environment oxygen content will be very limited because oxygen diffuses about 10,000 times more slowly through water than through air. [4]
In areas where oxygen is not present, soil microbes may use alternative electron acceptors such as nitrate, manganese, iron, sulfate, and carbon dioxide. An example of the result of the use of an alternative electron acceptor can be seen in figure 8.
Cation Exchange Capacity
Cation exchange capacity (CEC) is the ability of a soil to hold and exchange cations. The amount of CEC in soil is highly dependent on the texture and organic matter of the soil. The high surface area and negative charge of clay allows it to bind and exchange with soil solution, which contains cations that are important for plant and microbial health. [4]
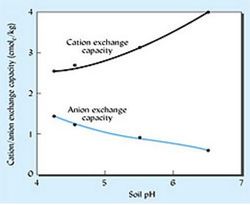
For many soils, especially those with low clay and organic carbon content , CEC is dependant upon soil pH (see figure 9). As pH decreases , an increasing amount of H+ ions are attracted to negatively charged clay particles and functional groups in SOM. This causes other cations, that were attached to these surfaces to fall off into the soil solution. CEC will increase as pH increases as less cations are being pushed out by H+ ions. [5]
At typical soil pH values (5-8), microbes and clay particles are both negatively charged, but microbes still to bind to clay. This binding could be due van der waals, hydrogen bonding, sharing electrons and ion exchange.
Redox Reactions
Reduction-oxidation (redox) reactions are chemical reactions in which reactants experience a change in oxidation number (which means these reactants either gain or lose electrons) . Many reactions in the soil involve the gain or loss of electrons, obtaining or releasing energy. Redox reactions are important in the soil, because microbes obtain energy through redox reactions for their metabolism, and the redox state can also determine the microbial processes that will occur.
Redox reactions include anabolism and catabolism process, both of which play important roles in microbial metabolism. Anabolism is the biosynthesis of cellular components, linked to energy requirements, while catabolism is the the biochemical processes leading to breakdown of organic substances, linked to energy production. For example, the photosynthesis and respiration processes are the coupled reactions in the soil, where plants required energy from light and reduce carbon dioxide to glucose, which then be used by microbes in the soil as the energy for their metabolism.
The tendency of compounds to accept or donate electrons is expressed as “redox potential”. Redox potential (Eh), determined from the concentration of oxidants and reductants in the environment, is to measure the oxidation-reduction status and electron availability within this system. Electrons are essential to all inorganic and organic chemical reactions. Redox potential measurements allow for rapid characterization of the degree of reduction and for predicting stability of various compounds that regulate nutrients and metal availability in soil and sediment. The inorganic oxidants include oxygen, nitrate, nitrite, manganese, sulfate, and CO2, while the reductants include various organic substrates and reduced inorganic compounds (NH4+, Fe2+, Mn2+, S2-, CH4, and H2). [15]
The redox potential of a substance depends on Affinity of molecules for electrons and Concentration of reductants and oxidants (referred to as redox pair) Anaerobic environments such as wetland soils and flooded soils are usually limited by electron acceptors and have an abundant supply of electron donors. In this case, most microbes’ activity is limited, and facultative and obligate microbes reduce the minerals following the electron tower. Aerated soils are usually limited by electron donors and have an abundant supply of electron acceptors (primarily O2). [15]
Salinity
Soil salinity refers to the salt content in the soil. The concentrations and types of ions in solution in the soil can cause modifications in the dispersion of the clay fraction, degrading the original soil fraction. The sodium ion, being monovalent, increases the width of the diffuse double layer on the surface of the clays, reducing the attractive forces between them with a consequent increase in particle dispersion. [16] The consequence of this dispersion of the clay is also shown by a reduction in stability of the soil aggregates, which are thus easily transported by rain or irrigation.
Soil salinization is a big problem for soils in arid or semi-arid regions and agricultural soils throughout the world. [5] Salts can adversely affect plant and microbial growth, due to destruction of the soil structure and its consequent compacting. The stress of high salt concentration can be detrimental for sensitive microorganisms and decrease the activity of surviving cells, due to the metabolic load imposed by the need for stress tolerance mechanisms.
Bioavailability
Bioavailability assesses what proportion of a contaminant present at a contaminated site is available for uptake by organisms. Bioavailability processes are the biological, chemical and physical processes that result in an organism being exposed to a contaminant present in the soil. These processes are: release of the contaminant from the solid phase, transport of the contaminant to and across a biological membrane and, incorporation into a living organism. [18]
Bioavailable molecules must cross a biological membrane, which means the molecules have to interact with the aqueous phase. Therefore, soil properties which control partitioning between the solid phase in soil and the pore water, such as pH, organic matter content, Eh, cation exchange capacity (CEC), and the concentration of clay minerals, have a significant impact on bioavailability. Increasing exchange sites aids the retention of molecules in the pore water in a bioavailable form, but molecules sorbed strongly to surfaces or in solid form are generally not bioavailable. [18]
Current Research
Soil functions are hugely influenced by soil aggregate properties like strength and porosity. The effect of soil management on soil aggregation can assist in determining best management practices. One study sought to quantify the long-term effect of rotation and tillage on aggregate shape , strength and pore characteristics. [8] Soil samples were taken from a long-term rotation and tillage trial with both continuous and diverse rotations. The size of these soil samples were determined by X-ray micro-CT, and the study concluded several findings :
- Rotation and tillage effect on aggregate and bulk soil properties
- Rotation affected soil aggregate properties more than tillage.
- Tillage have a stronger effect on bulk soil porosity
References
1. "Soil_Microaggregate_and_bacteria." UC Berkeley, n.d. Web. 25 Feb. 2016. <http://em-lab.berkeley.edu/EML/images/TEM-Gallery1/pages/Soil_Microaggregate_and_bacteria.htm>.
2. " Soil Reaction (pH)." University of New South Wales, n.d. Web. 25 Feb. 2016. <http://www.terragis.bees.unsw.edu.au/terraGIS_soil/sp_soil_reaction_ph.html>.
3. "Soil Images - Redoximorphic Features." New England Soil, n.d. Web. 25 Feb. 2016. <http://nesoil.com/images/redox.htm>.
4. Sylvia, D. M. Principles and Applications of Soil Microbiology. Upper Saddle River, NJ: Pearson Prentice Hall, 2005. Print.
5. Brady, Nyle C., and Ray R. Weil. Elements of the Nature and Properties of Soils. Upper Saddle River, NJ: Pearson Prentice Hall, 2010. Print.
6. Hamarshid N.H., Othman M.A., and Hussain M.-A.H., 2010. Effects of soil texture on chemical compositions, microbial populations and carbon mineralization in soil. Egypt. J. Exp. Biol. (Bot.), 6(1), 59-64. Kabala C. and Zapart J., 2012.
7. Todar, Kenneth. "Nutrition and Growth of Bacteria." N.p., n.d. Web. 13 Mar. 2016. <http://textbookofbacteriology.net/nutgro_5.html>.
8. Munkholm, L. J., Heck, R. J., Deen, B., & Zidar, T. (2016). Relationship between soil aggregate strength, shape and porosity for soils under different long-term management. Geoderma, 26852-59. doi:10.1016/j.geoderma.2016.01.005
9. "PH and Organic Substrate Nutrients." N.p., n.d. Web. 13 Mar. 2016. <http://organicsoiltechnology.com/ph-and-organic-substrate-nutrients.html>.
10. Fierer, N., and R. B. Jackson. "The Diversity and Biogeography of Soil Bacterial Communities." Proceedings of the National Academy of Sciences 103.3 (2006): 626-31. Web.
11. "Soil Structure and Macropores." Soil Quality: Indicators:. NRCS, n.d. Web. 13 Mar. 2016. <http://soilquality.org/indicators/soil_structure.html>.
12. "Electron Tower Theory." Bioremediation Specialists, n.d. Web. 14 Mar. 2016. <http://bioremediation-specialists.com/Electron-Tower-Theory.php>.
13. Moravec, C., Whiting, D., and Reeder, J. “Introduction to Soils.” CMG GardenNotes. Colorado State University Extension. Oct. 2015. <http://www.cmg.colostate.edu/>
14. “BIO 212 Study Guide”. Iowa State University. <https://www.studyblue.com/notes/note/n/bio-212-study-guide-2012-13-yin/deck/9716305>
15. Delaune, R. D., and Reddy, K, R., Encyclopedia of Soils in the Environment. 2005, Elsevier Ltd.
16. Maganhotto, C. M.,and Francisconi Fay,E., (2012). Effect of Salinity on Soil Microorganisms, Soil Health and Land Use Management, Dr. Maria C. Hernandez Soriano (Ed.), InTech, Available from: http://www.intechopen.com/books/soil-health-and-land-usemanagement/Effect-of-salinity-on-soil-microorganisms
17. Provin, T., and Pitt, J. L. “Managing Soil Salinity”. Texas AgriLife Extension Service. March, 2012. <http://soiltesting.tamu.edu/publications> Edited by students of Kate Scow
18. Hodson, M. E., Vijver, M. G. and Peijnenberg, W. J. G. M. (2011) Bioavailability in soils. In: Swartjes, F. A. (ed.) Dealing with contaminated sites: from theory towards practical application. Springer, pp. 721-746. ISBN 9789048197569 doi: 10.1007/978-90-481-9757-6 Available at http://centaur.reading.ac.uk/20839/