Soil Health: Difference between revisions
| Line 86: | Line 86: | ||
One of the major limitations of existing indicators is that they pay relatively little attention to the key role of soil microorganisms in soil health. The FAO, for example, does not have any microbe-focused indicators in its “Biological and Chemical Indicators,” even though its definition of soil health emphasizes the importance of soil biology. The latest draft of “Biological Indicators and Soil Functions” published by the NRCS is more comprehensive, including parameters such as soil enzyme activity, particulate organic matter (POM), potentially mineralizable nitrogen (PMN), phospholipid fatty acid (PLFA) analysis, and respiration. Β-glucosidase activity may be particularly important among soil enzymes measured because of its role in plant residue degradation.<ref>Lehman, R. M., Cambardella, C. A., Stott, D. E., Acosta-Martinez, V., Manter, D. K., Buyer, J. S., … Karlen, D. L. (2015). Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability, 7(1), 988–1027. http://doi.org/10.3390/su7010988</ref> | One of the major limitations of existing indicators is that they pay relatively little attention to the key role of soil microorganisms in soil health. The FAO, for example, does not have any microbe-focused indicators in its “Biological and Chemical Indicators,” even though its definition of soil health emphasizes the importance of soil biology. The latest draft of “Biological Indicators and Soil Functions” published by the NRCS is more comprehensive, including parameters such as soil enzyme activity, particulate organic matter (POM), potentially mineralizable nitrogen (PMN), phospholipid fatty acid (PLFA) analysis, and respiration. Β-glucosidase activity may be particularly important among soil enzymes measured because of its role in plant residue degradation.<ref>Lehman, R. M., Cambardella, C. A., Stott, D. E., Acosta-Martinez, V., Manter, D. K., Buyer, J. S., … Karlen, D. L. (2015). Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability, 7(1), 988–1027. http://doi.org/10.3390/su7010988</ref> | ||
[[File:23 micro biomass.png| | [[File:23 micro biomass.png|frame|border|right|top|upright|alt=Alt|Measuring microbial biomass a biologically-focused indicator of soil health integrates physical, chemical, and biological indicators.]] | ||
'''What could additional indicators of soil health look like?''' | '''What could additional indicators of soil health look like?''' | ||
Revision as of 05:43, 15 March 2016
Introduction
“Essentially, all life depends upon the soil...There can be no life without soil and no soil without life; they have evolved together."[1]
The Living Soil
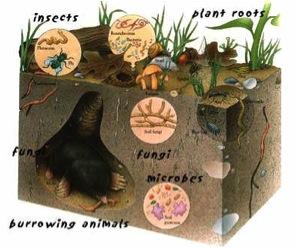
Much like the success of the human body is measured by its health, soil health is a measure of a complex set of biological, chemical and physical interactions, which are driven by microbial processes. The soil supports and sustains most life forms on earth, but it is the work of microorganisms that give soil its unique life giving properties.
It is estimated that between 1-5% of all organic matter in the soil comes from microorganism. [3] In just one teaspoon of healthy soil more microbial life exists than the current global population.[4] Today, less than 1% of the bacteria, fungi and protozoa that help support our global population are identified as well as the crucial roles they play in supporting soil health.
Healthy soils are being lost at alarming rates through degradation, salinization, acidification, desertification, erosion, nutrient depletion and microbial species extermination. We have less and less healthy land available, yet we are being pressured to feed a growing population. Having an understanding of the processes microbes undergo which support the formation and maintenance of healthy soils is essential for facing the global food security challenges of the present and future.[5]
This page will explore ideas and research relating soil health and microbiology to the agricultural context.
Soil Quality vs. Soil Health
Soil quality and soil health are two related but distinguishable terms. Delineating between soil quality and soil health is useful for clarifying the complex and somewhat amorphous definition of soil health.
Soil quality, generally speaking, refers to how functional a soil is as defined by human goals. Within the agricultural context, soil quality refers to the quality or quantity of crop yield that a soil can produce within a given production system.
Soil health, in contrast to soil quality, takes into account other ecosystem functions and services that go beyond immediate human use goals for a particular soil. The term soil health was introduced in order to integrate a more holistic lens to the understanding of the complex network of relationships that exist within soils, including the roles of microbes. Additionally, it focuses on the mitigation and reversal of soil degradation globally.
A healthy agricultural soil, for example, is not simply a soil that acts as a substrate for high quality crop production; it will also support a diversity of organisms, which will perform functions such as nutrient cycling, carbon sequestration, degradation of toxins, and improving soil structure for water retention and aeration. All of these functions support healthy plant growth; therefore the human functionality of the soil is incorporated into the term “soil health.”
Definitions and Indicators
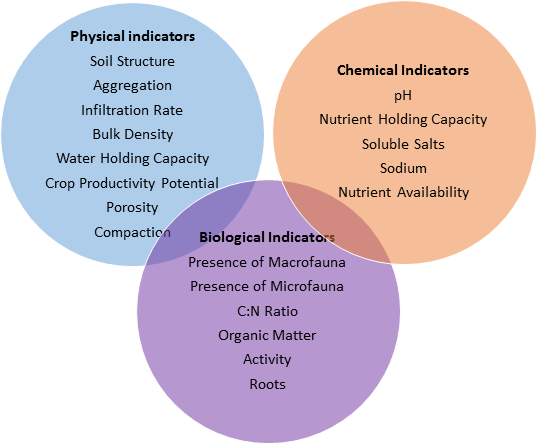
If soil health is a measure of a complex set of biological, chemical and physical interactions, then it must be measured by an assessment of many indicators taken together, rather than by a single parameter. Indicators can fall into the above-mentioned categories (biological, chemical, physical). Most of the indicators of soil health that exist today (e.g. FAO, NRCS) can either be directly linked to microbial processes or are affected by them.
FAO
The FAO takes a global approach to soil health and maintains databases of soil health assessments for soils worldwide.[6] Soils can be assessed using either an absolute framework or a relative framework. The absolute definition of soil health compares a given soil to a set of ideal properties, whereas the relative definition takes into account the current use of that soil. A degraded soil under sparse vegetation might have low soil health on the absolute scale, but may score well on the relative scale since the soil properties support the existing ecosystem.[7]
FAO indicators are divided into two distinct categories, “Physical” and “Biological and Chemical.” Although the FAO divides physical from biological indicators, many physical indicators of soil health have a biological component. According to the FAO, the three most important physical indicators of soil health are:
(1)“the absence of sealing and crusting” (2)“the absence of erosion both by water and by wind” (3)“the absence of compaction”[8]
Sealing occurs mainly in soils covered by infrastructure. Crusting however, depends in part, on aggregate stability, and therefore microbiological processes in the soil. Erosion by wind and water could be influenced by microbial activity as well, since soils with well-formed aggregates are less likely to erode.[9] Compaction results primarily from heavy livestock grazing and machinery and could alter microbial activity by decreasing gas diffusion and water infiltration).[10]
Biological and chemical indicators of soil health described by the FAO include: (1) “soil nutrient mining” (2) “salinization” (3) “pollution”[11]
Nutrient depletion is only considered applicable in agricultural contexts and results from management practices that do not restore nutrients removed by crops. Limitation of key soil nutrients such as nitrogen or phosphorus may restrict microbial growth.[12] Salinization can be a consequence of poor irrigation management, and can have detrimental effects on microbial biomass and activity.[13] Pollution resulting from inputs of heavy metals, industrial chemicals, or excessive fertilization, for example, could harm microbial communities[14] but could also represent an opportunity for bioremediation using microbes capable of metabolizing the pollutants.[15]
NRCS
The NRCS “Guidelines for Soil Quality Assessment in Conservation and Planning”[16] provides an outline for a nine-step planning process for incorporating soil quality and health indicators into the conservation planning process. In these guidelines, a healthy soil is defined as one that should perform the following functions:
- sustain biological activity, diversity, and productivity;
- regulate and partition water and solute flow;
- filter and buffer, degrade, immobilize, and detoxify organic and inorganic materials, including industrial and municipal by-products and atmospheric deposition;
- store and cycle nutrients and other elements within the earth’s biosphere; and
- provide support of socioeconomic structures and protection for archaeological treasures associated with human habitation.
The NRCS has created a list of clearly delineated, measurable properties (or indicators) that, taken together, can be used to assess the healthy functioning of a soil according to the definition above. Many, if not all, of the NRCS indicators can be directly linked to microbial activity in the soil. "Indicator sheets" define each indicator, its significance, and standardized measurement methods. Examples of some key indicators are featured below with links to their indicator sheets, to illustrate how microbial processes are drivers of these soil health indicators.
Physical Properties
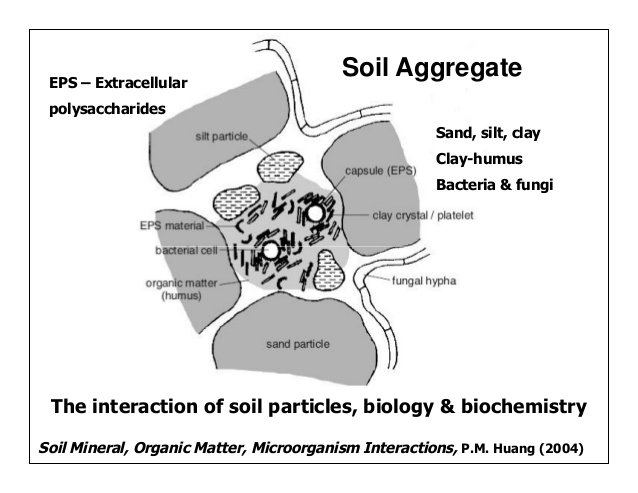
Aggregate stability (PDF, 380KB[1]) is enhanced and accelerated by the activity of many different groups of soil microorganisms. Fungal hyphae bind strongly to inorganic soil particles (like clays) and thus physically connect soil particles that were not previously grouped into an aggregate.[17] Mycorrhizal fungi produce glycoproteins (such as glomalin) that bind soil particles together and are thus highly correlated with aggregate stability.[18][19] Bacteria also contribute to aggregate stability; many bacteria either actively excrete extracellular polymeric substances or release them during cell lysis and death.[20] The glucose content of these polymeric substances is correlated with soil aggregation.[21] Interestingly, the addition of these compounds alone to sterilized soil does not generally increase soil aggregation, suggesting that other microbial processes and transformations are necessary for these polymeric substances to contribute to aggregate stability and soil structure.[22]
Soil Structure and Macropores (PDF, 480KB[2]) are established by soil macroaggregates and are thus influenced by microorganisms in the same way as aggregate stability.[23] Many types of microorganisms excrete substances that contribute to aggregate formation or release them during the turnover of microbial biomass. This in turn affects the development of soil structure and macropores.[24][25] Macropores can also be created by soil macrofauna, such as earthworms and arthropods, as they move through the soil.[26]
Chemical Properties
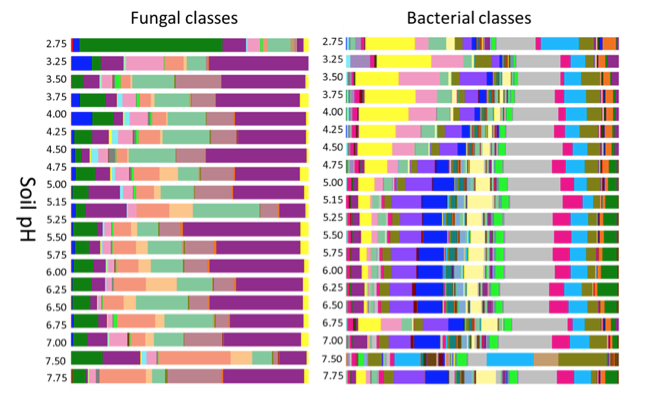
Soil pH (PDF, 265KB[3]) both impacts and is impacted by biological, chemical, plant, and microbial activity thresholds, all of which will critically affect plant available nutrients and N and P loss/retention. Microorganisms can alter pH by producing organic acids as a byproduct of fermentation or through any number of oxidation-reduction (redox) reactions.[27] For example, the oxidation of both ammonium (to nitrate) and sulfur (to sulfate) produces protons (H+) and thus acidifies the soil.[28] This in turn affects the distribution of microorganisms - fungi in particular are quite tolerant of acid soils.[29]
Soil Nitrate (PDF, 673KB[[4]]) is one inorganic pool of nitrogen (N). Nitrogen represents a critical (and often limiting) nutrient for both plants and soil microbes. Nitrate (NO3) is introduced into the soil through plant residues, fertilizer inputs, and sometimes lightning. Microbial processes drive the nitrogen cycle and both produce NO3 (through nitrification) and consume it (through denitrification and immobilization).[30]
Biological Properties
Particulate Organic Matter (PDF, 1.8MB[[5]]) can boost nutrient retention, improve soil structure, increase water infiltration, and help prevent soil erosion. Leaf litter and other large scale organic inputs (dead trees, manure, dead animals, etc.) are integrated into the particulate organic matter pool as they are degraded by a large consortium of soil microbes(“Soil Quality Indicator Sheets | NRCS Soils,” n.d.)
Potentially Mineralizable Nitrogen (PDF, 611KB[[6]]), or PMN, indicates soil productivity and N supplying potential, and also directly enhances soil microbial activity.[31] Mineralization is the conversion of organic N to inorganic N, often as a result of microbes secreting N that is in excess of their own requirements.[32] As mineralized nitrogen is subsequently nitrified by bacteria and archaea, determination of PMN requires flooded anaerobic conditions in order to inhibit the aerobic process of nitrification.[33]
Soil Enzymes (PDF, 220KB[[7]]) are produced by all the plants and microbes that live in the soil. These enzymes work to break down plant residues and other organic inputs and release nutrients back into the soil environment.[34]
Soil Respiration (PDF, 329KB[[8]]) refers to the CO2 released from the bulk soil and is mainly the product of aerobic soil microorganisms decomposing soil organic matter and contributing to soil organic carbon.[35]
Total Organic Carbon (PDF, 210KB[[9]]) is essentially the carbon stored in soil organic matter and it is released from those stores primarily by the activity of microorganisms that decompose organic matter. Carbon stores in the soil provide the main source of energy to both plants and soil microbes.[36]
Beyond Existing Indicators: Incorporating Soil Biology
One of the major limitations of existing indicators is that they pay relatively little attention to the key role of soil microorganisms in soil health. The FAO, for example, does not have any microbe-focused indicators in its “Biological and Chemical Indicators,” even though its definition of soil health emphasizes the importance of soil biology. The latest draft of “Biological Indicators and Soil Functions” published by the NRCS is more comprehensive, including parameters such as soil enzyme activity, particulate organic matter (POM), potentially mineralizable nitrogen (PMN), phospholipid fatty acid (PLFA) analysis, and respiration. Β-glucosidase activity may be particularly important among soil enzymes measured because of its role in plant residue degradation.[37]
What could additional indicators of soil health look like? Soil microbial biomass has been suggested as one alternative.[38] Because microorganisms are involved in complex, interrelated processes that drive soil health, measuring total microbial biomass gives a more complete picture of soil health than measuring individual enzymes or particular species. However, care should be taken to measure changes from a baseline for a given soil rather than total microbial biomass, since comparing totals across soils could be misleading.
A large number of currently available potential biological indicators were analyzed based on input from experts and stakeholders and ranked them according to a number of criteria. Their ranked list can be found in Table 6 in Ritz et al. (2009).[39] Top-ranked indicators included: (1) terminal restriction fragment length polymorphism (TRFLP) characterization of ammonia oxidizers/denitrifiers, (2) PLFA profiles of the entire soil microbial community, (3) TRFLP of the intergenic spacer (ITS) region of fungal rRNA, and many more. Some of the indicators were most useful for one specific function such as nitrogen or carbon cycling, whereas others could be used as a proxy for multiple functions.[40]
New possible evaluation techniques for soil biological health might also one day include genetic tools. Since the 1980’s, when microbiologists began to acknowledge that organisms identified by pure-culture techniques represented only a tiny fraction of the metabolic and organismal diversity existing in the world, many new technologies have been developed in order to address the pressing question of not only “who is there” in the microbial community (which can be addressed using techniques like PLFA), but also, “what are they doing”.[41]
Metagenomics is one such method for genomic analysis of communities of organisms. The word itself was meant to convey its usefulness in analyzing a collection of similar, but not identical items. Metagenomics involves isolating DNA from a particular environmental sample, cloning the DNA into a suitable vector, generating a host bacterium from cloned DNA, and finally screening the resulting transformants. Using diverse methods to analyze gene expression in the generated hosts, it has been possible to establish the function of certain genes. These new insights, however, taken out of specific biological context, cannot truly be used to make any conclusions at the ecological scale. The current state of the technology still requires some technical advances in order to move the field forward from concluded inferences to a true mechanistic analysis and understanding of functional genes.[42][43]
Soil Health and Agriculture
Soil is an interconnected system with high levels of exchange of energy between organisms and physical-chemicals components, which leads to the concept that soil is a self-organized system. However, despite having the unique and incredible capacity of adapting to environmental change, described as resistance and resilience, microbes are sensitive to land management and climate change. Resistance is the capacity of the soil to maintain its health despite the magnitude of the change caused by any kind of perturbation. Resilience is the capacity of the system to return to its original state after a disturbance, which is also known as the self-healing capacity of the system.[44] Some impacts of soil management in soil health are described below:
Physical Disturbances
Tillage
Disturbance of the soil through tillage breaks aggregates, destroying soil structure and exposing microbes to the environment. Tillage usually results in high mortality within the microbial community and/or a drastic decrease in activity. Without microbial activity, no organic matter is mineralized. Additionally, the use of heavy machinery for tillage can compact the soil, preventing infiltration of water and gases. Compacted soils can, in some cases, form a crust on the surface, which seals the soil, prevents plant and microbial growth, and increases runoff.[45]
Overgrazing
The intense biological disturbance of soil caused by overgrazing cattle can be equally detrimental to soil. In an overgrazed pasture, the most palatable species are consumed first, which favors the growth of weedy species. An exposed and compacted soil will have a higher temperature and diminished habitat for the soil microbes. As a result, there is a higher necessity of synthetic inputs, such as fertilizers and pesticides to control those weeds.[46]
Inputs
Fertilizers
The addition of inorganic fertilizers into the soil in an attempt to increase productivity impacts microbial activity in the short-term by reducing its biomass, as high application rates increase the soil's osmotic potential, which can be toxic for microbes. However, when applied in a well-managed agroecosystem (where crop residues are kept in the soil as a carbon source), mineral fertilizers can supply microbes with nutrients (N, P and K) and stimulate microbial activity in the long-term.[47] Additionally, excess application of fertilizers can lead to leaching and eutrophication in water bodies.[48]
Fumigation
The use of biocides for pest control is highly effective in the short-term, but in the long term pathogens can recolonize. Fumigation destroys predator-prey relationships which exist within the natural food web, allowing pathogens to grow without predator control.
Water Availability
Exposed soil
An exposed soil has high level of water evaporation. Without water, the microbial population dies. Plant productivity and the symbiotic relationship between plants and microbes, such as those found in the rhizosphere, are also diminished. An exposed soil is also subject to increased temperature and a loss in water retention, both of which increase erosion by runoff.
Irrigation Practices
Irrigation allows plants to keep stomata open and maximize transpiration, which increases productivity and thus C inputs into the soil, while also providing moisture that is essential for microbial activity. It is estimated that 17% of land that is irrigated produces 40% of the global food.[49] Irrigation affects the distribution of water in the soil profile, which in turn affects the distribution of oxygen, and can create anaerobic micro-environments. The use of low quality irrigation water, failure to add enough water to leach salts from the soil profile, and the presence of a shallow groundwater table can all lead to salinization in arid and semi-arid environments.[50][51] Salinization affects microbes directly by increasing the osmotic potential of the soil solution, as well as indirectly by affecting plant growth and C cycling.
Crop Rotation
Monoculture
Planting a homogeneous stand of a single crop can select a narrow set of microbial species, which can be more vulnerable for pest cycles. High microbial abundance within few trophic groups does not guarantee soil health unless it includes key groups that provide certain functions.[52] However, even when crops are rotated, some nutrients (such as nitrogen) and organic matter are exhausted, demanding more fertilizer inputs. Rotating crops does, however, provides windows where the farmer can incorporate organic inputs which would contribute to soil organism diversity.
Mitigation
Unfortunately, approximately 40% of the world’s cropland has become unproductive due to erosion, which has reduced food production and contributed to the undernourishment of more than 3 billion people.[53] This degradation process may be exacerbated by climate change, creating a positive feedback loop, where climate change accelerates soil degradation and soil degradation stimulates climate change. For example, when a soil system is disturbed by tillage, enhanced microbial degradation of organic matter may lead to increased losses of mineralized N through leaching or volatilization, especially when the supply of mineralized nitrogen exceeds biological demand. These processes increase greenhouse gas emissions leading to higher temperatures, subsequently, greater temperatures promulgate faster chemical reactions.[54]
New approaches, such as climate-smart agriculture and agro-ecology, have been suggested to make agriculture more adaptive and resilient to environmental pressures, while simultaneously reducing its own negative impacts on ecosystem services. Studies have shown that sustainable soil management could produce up to 58% more food.[55] Soil management practices that promote soil health include:
- cover crops
- no-till farming
- compost and other organic fertilizers which include C in addition to other macro and micro nutrients.
- diversified cropping systems
- biological pathogen control[56]
Microbes and Soil Disease Suppression: A Case for Soil Health
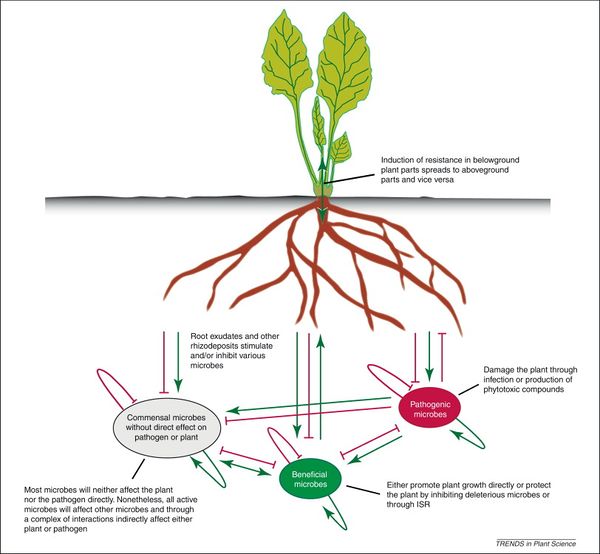
Soil health in agricultural systems must take into account plant health. The rhizosphere is the soil zone around plant roots where microbially mediated soil-plant interaction occurs. Plants live in close association with rhizosphere microbes, which are referred to as the plant’s “second genome”, similar to the human gut.[57] Through long-term adaptation and coevolution, plant roots and their soil microbiome have developed complex interrelationships and symbioses. One of these processes is the suppression of soil plant pathogens by microbial populations.
The microbial population in the rhizosphere is abundant and highly diverse. Such diversity contributes to “niche overlap”; in effect, the native microbes leave no empty niche for pathogens to colonize plant roots. Furthermore, fierce competition for a limited resource base ensures that no single microbial group can dominate.[58] Through these mechanisms, microbes inhibit plant pathogens and create natural soil disease suppression.
Application of pesticides, fungicides, and inorganic fertilizers applies selective pressure to the root microbiome, reducing abundance and diversity of soil microorganisms. This creates space for newcomers that can be harmful or beneficial to plants. For example, when a mycorrhizal fungi population is inhibited by fungicide, pathogenic invaders enter the “empty” rhizosphere niche and infect plant roots. Evidence suggests that plants select for beneficial microbes through secretion of root exudates.[59] In this case, a less diverse root microbiome increases soil disease suppression. Ongoing research is being done on the effect of intentionally disrupting plant-microbe associations to treat pathogen-infected soils. In one study,[60] diseased agricultural soils due to successive monocrops were inoculated with a microbe pathogen antagonist. Recruiting microbes to attack soil pathogens as a means facilitating soil disease suppression and plant health is at the forefront of research advances.
Current Research
The study of soil health represents an exciting and rapidly growing body of science. Many major universities and agencies have dedicated significant time and resources to further understand the role microorganisms play in creating and maintaining soil health. Explore these pages to see some of the work being done by some of the top labs in the country:
- Yarwood Microbial Ecology and Biogeochemistry Lab, University of Maryland
- Scow Microbial Ecology Lab, University of California Davis
- Fierer Lab, University of Colorado
- and Agriculture Organization of the United Nations (UN-FAO)
- International Year of Soils (UN-FAO)
- States Department of Agriculture (USDA)
- Resources Conservation Service (NRCS)
- Science Society of America (SSSA)
References
- ↑ USDA Yearbook of Agriculture. (1938). 75th Congress, 2d session. House Document No. 398. Retrieved from http://naldc.nal.usda.gov/download/IND50000140/PDF<ref/
- ↑ Fresh, Organic Produce & Mushrooms for Sale in Porter County IN | Living Earth Farms. (n.d.). Retrieved from http://www.livingearthfarms.net/
- ↑ The Living Soil. (n.d.). Retrieved March 13, 2016, from http://www.ext.colostate.edu/mg/gardennotes/212.html
- ↑ USDA NRCS. (n.d.). Soil Health Nuggets. Retrieved March 13, 2016, from http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb1101660.pdf
- ↑ Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., … Snyder, P. K. (2005). Global Consequences of Land Use. Science, 309(5734), 570–574. http://doi.org/10.1126/science.1111772
- ↑ FAO. (2016b). Global Soil Health Indicators and Assessment. Retrieved February 21, 2016, from http://www.fao.org/soils-portal/soil-degradation-restoration/global-soil-health-indicators-and-assessment/en/
- ↑ FAO. (2016a). Global Soil Health. Retrieved February 21, 2016, from http://www.fao.org/soils-portal/soil-degradation-restoration/global-soil-health-indicators-and-assessment/global-soil-health/en/
- ↑ FAO. (2016c). Management and Natural Processes affecting the biological and chemical aspects of soils. Retrieved February 21, 2016, from http://www.fao.org/soils-portal/soil-degradation-restoration/global-soil-health-indicators-and-assessment/soil-heath-biological-and-chemical/en/
- ↑ LeBissonnais, Y. (1996). Aggregate stability and assessment of soil crustability and erodibility .1. Theory and methodology. European Journal of Soil Science, 47(4), 425–437.
- ↑ Arias, M. E., Gonzalez-Perez, J. A., Gonzalez-Vila, F. J., & Ball, A. S. (2005). Soil health - a new challenge for microbiologists and chemists. International Microbiology, 8(1), 13–21.
- ↑ FAO. (2016b). Global Soil Health Indicators and Assessment. Retrieved February 21, 2016, from http://www.fao.org/soils-portal/soil-degradation-restoration/global-soil-health-indicators-and-assessment/en/
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.page
- ↑ Wichern, J., Wichern, F., & Joergensen, R. G. (2006). Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma, 137(1-2), 100–108. http://doi.org/10.1016/j.geoderma.2006.08.001
- ↑ Brookes, P. C. (1995). The use of microbial parameters in monitoring soil pollution by heavy metals. Biology and Fertility of Soils, 19(4), 269–279. http://doi.org/10.1007/BF00336094
- ↑ Samanta, S. K., Singh, O. V., & Jain, R. K. (2002). Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends in Biotechnology, 20(6), 243–248. http://doi.org/10.1016/S0167-7799(02)01943-1
- ↑ USDA NRCS. (2001). Guidelines for Soil Quality Assessment in Conservation Planning. Retrieved from http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051259.pdf
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.page
- ↑ Wright, S. F., & Upadhyaya, A. (n.d.). A survey of soils for aggregate stability and glomalin, a glycoprotein produced by the hyphae of arbuscular mycorrhizal fungi. Plant and Soil, 198, 97–107.
- ↑ Wu, Q., Cao, M., Zou, Y., & He, X. (2014). Direct and indirect effects of glomalin, mycorrhizal hyphae, and roots on aggregate stability in rhizosphere of trifoliate orange. Scientific Reports, 4, 5823.
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.page
- ↑ Martens, D. A., & Frankenburger Jr., W. T. (1992). Decomposition of bacterial polymers in soil and their influence on soil structure. Biology and Fertility of Soils, 13, 65–73.
- ↑ Martens, D. A., & Frankenburger Jr., W. T. (1992). Decomposition of bacterial polymers in soil and their influence on soil structure. Biology and Fertility of Soils, 13, 65–73.
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.
- ↑ Wright, S. F., & Upadhyaya, A. (n.d.). A survey of soils for aggregate stability and glomalin, a glycoprotein produced by the hyphae of arbuscular mycorrhizal fungi. Plant and Soil, 198, 97–107.
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.
- ↑ Soil Quality Indicator Sheets | NRCS Soils. (n.d.). Retrieved March 13, 2016, from http://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/health/assessment/?cid=stelprdb1237387
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.
- ↑ Soil Quality Indicator Sheets | NRCS Soils. (n.d.). Retrieved March 13, 2016, from http://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/health/assessment/?cid=stelprdb1237387
- ↑ Soil Quality Indicator Sheets | NRCS Soils. (n.d.). Retrieved March 13, 2016, from http://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/health/assessment/?cid=stelprdb1237387
- ↑ Sylvia, D. M., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (2005). Principles and Applications of Soil Microbiology (2nd ed.). Upper Saddle River, NJ: Pearson Prentice Hall. Retrieved from http://www.pearsonhighered.com/educator/product/Principles-and-Applications-of-Soil-Microbiology/9780130941176.
- ↑ Waring, S. A., & Bremner, J. M. (1964). Ammonium production in soil under waterlogged conditions as an index of nitrogen availability. Nature, 201, 951–952.
- ↑ Soil Quality Indicator Sheets | NRCS Soils. (n.d.). Retrieved March 13, 2016, from http://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/health/assessment/?cid=stelprdb1237387
- ↑ Soil Quality Indicator Sheets | NRCS Soils. (n.d.). Retrieved March 13, 2016, from http://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/health/assessment/?cid=stelprdb1237387
- ↑ Soil Quality Indicator Sheets | NRCS Soils. (n.d.). Retrieved March 13, 2016, from http://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/health/assessment/?cid=stelprdb1237387
- ↑ Lehman, R. M., Cambardella, C. A., Stott, D. E., Acosta-Martinez, V., Manter, D. K., Buyer, J. S., … Karlen, D. L. (2015). Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability, 7(1), 988–1027. http://doi.org/10.3390/su7010988
- ↑ Gonzalez-Quinones, V., Stockdale, E. A., Banning, N. C., Hoyle, F. C., Sawada, Y., Wherrett, A. D., … Murphy, D. V. (2011). Soil microbial biomass-Interpretation and consideration for soil monitoring. Soil Research, 49(4), 287–304. http://doi.org/10.1071/SR10203 H
- ↑ Ritz, K., Black, H. I. J., Campbell, C. D., Harris, J. A., & Wood, C. (2009). Selecting biological indicators for monitoring soils: A framework for balancing scientific and technical opinion to assist policy development. Ecological Indicators, 9(6), 1212–1221. http://doi.org/10.1016/j.ecolind.2009.02.009
- ↑ Ritz, K., Black, H. I. J., Campbell, C. D., Harris, J. A., & Wood, C. (2009). Selecting biological indicators for monitoring soils: A framework for balancing scientific and technical opinion to assist policy development. Ecological Indicators, 9(6), 1212–1221. http://doi.org/10.1016/j.ecolind.2009.02.009
- ↑ Handelsman, J. (2004). Metagenomics: Application of Genomics to Uncultured Microorganisms. Microbiology and Molecular Biology Reviews, 68(4), 669–685. http://doi.org/10.1128/MMBR.68.4.669-685.2004
- ↑ Handelsman, J. (2004). Metagenomics: Application of Genomics to Uncultured Microorganisms. Microbiology and Molecular Biology Reviews, 68(4), 669–685. http://doi.org/10.1128/MMBR.68.4.669-685.2004
- ↑ Rondon, M. R., August, P. R., Bettermann, A. D., Brady, S. F., Grossman, T. H., Liles, M. R., … Goodman, R. M. (2000). Cloning the Soil Metagenome: a Strategy for Accessing the Genetic and Functional Diversity of Uncultured Microorganisms. Applied and Environmental Microbiology, 66(6), 2541–2547. http://doi.org/10.1128/AEM.66.6.2541-2547.2000
- ↑ Geisseler, D., & Scow, K. M. (2014). Long-term effects of mineral fertilizers on soil microorganisms – A review. Soil Biology and Biochemistry, 75, 54–63. http://doi.org/10.1016/j.soilbio.2014.03.023
- ↑ Geisseler, D., & Scow, K. M. (2014). Long-term effects of mineral fertilizers on soil microorganisms – A review. Soil Biology and Biochemistry, 75, 54–63. http://doi.org/10.1016/j.soilbio.2014.03.023
- ↑ Bot, A., & Benites, J. (2005). The importance of soil organic matter: key to drought-resistant soil and sustained food production. Food & Agriculture Org. Retrieved from https://books.google.com/books?hl=en&lr=&id=dJe5-pmmjaAC&oi=fnd&pg=PP10&dq=Bot,+A.,+%26+Benites,+J.+(2005).+The+importance+of+soil+organic+matter.+FAO+Soils+Bulletin.+doi:10.1080/03650340214162&ots=FvzYW77rvl&sig=2pWepjUk315bvm-fx55R-_kcfYM
- ↑ Geisseler, D., & Scow, K. M. (2014). Long-term effects of mineral fertilizers on soil microorganisms – A review. Soil Biology and Biochemistry, 75, 54–63. http://doi.org/10.1016/j.soilbio.2014.03.023
- ↑ Ongley, E. D. (1996). Control of water pollution from agriculture. Food & Agriculture Org. Retrieved from https://books.google.com/books?hl=en&lr=&id=LxJsTnFoGagC&oi=fnd&pg=PA3&dq=Ongley,+E.+D.+(1996).+Chapter+3.+Fertilizers+as+water+pollutants.+Control+of+Water+Pollution+from+Agriculture.+FAO+Irrigation+and+Drainage+Paper+55,+37%E2%80%9352&ots=AS4mBIzA69&sig=IddpgpuSM8GgbvwXPdb-Yhko4Aw
- ↑ THE STATE OF FOOD AND AGRICULTURE 2002. (n.d.). Retrieved March 14, 2016, from http://www.fao.org/docrep/004/y6000e/y6000e00.htm
- ↑ Ongley, E. D. (1996). Control of water pollution from agriculture. Food & Agriculture Org. Retrieved from https://books.google.com/books?hl=en&lr=&id=LxJsTnFoGagC&oi=fnd&pg=PA3&dq=Ongley,+E.+D.+(1996).+Chapter+3.+Fertilizers+as+water+pollutants.+Control+of+Water+Pollution+from+Agriculture.+FAO+Irrigation+and+Drainage+Paper+55,+37%E2%80%9352&ots=AS4mBIzA69&sig=IddpgpuSM8GgbvwXPdb-Yhko4Aw
- ↑ Podmore, C. (2009). Irrigation salinity–causes and impacts. Primefact, 937(1), 1–4.
- ↑ Geisseler, D., & Scow, K. M. (2014). Long-term effects of mineral fertilizers on soil microorganisms – A review. Soil Biology and Biochemistry, 75, 54–63. http://doi.org/10.1016/j.soilbio.2014.03.023
- ↑ Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., … Snyder, P. K. (2005). Global Consequences of Land Use. Science, 309(5734), 570–574. http://doi.org/10.1126/science.1111772
- ↑ Geisseler, D., & Scow, K. M. (2014). Long-term effects of mineral fertilizers on soil microorganisms – A review. Soil Biology and Biochemistry, 75, 54–63. http://doi.org/10.1016/j.soilbio.2014.03.023
- ↑ FAO. (2016a). Global Soil Health. Retrieved February 21, 2016, from http://www.fao.org/soils-portal/soil-degradation-restoration/global-soil-health-indicators-and-assessment/global-soil-health/en/
- ↑ FAO. (2016b). Global Soil Health Indicators and Assessment. Retrieved February 21, 2016, from http://www.fao.org/soils-portal/soil-degradation-restoration/global-soil-health-indicators-and-assessment/en/
- ↑ Berendsen, R. L., Pieterse, C. M. J., & Bakker, P. A. H. M. (2012). The rhizosphere microbiome and plant health. Trends in Plant Science, 17(8), 478–486. http://doi.org/10.1016/j.tplants.2012.04.001
- ↑ Ghorbani, R., Wilcockson, S., Koocheki, A., & Leifert, C. (2008). Soil management for sustainable crop disease control: a review. Environmental Chemistry Letters, 6(3), 149–162. http://doi.org/10.1007/s10311-008-0147-0
- ↑ Berendsen, R. L., Pieterse, C. M. J., & Bakker, P. A. H. M. (2012). The rhizosphere microbiome and plant health. Trends in Plant Science, 17(8), 478–486. http://doi.org/10.1016/j.tplants.2012.04.001
- ↑ Rondon, M. R., August, P. R., Bettermann, A. D., Brady, S. F., Grossman, T. H., Liles, M. R., … Goodman, R. M. (2000). Cloning the Soil Metagenome: a Strategy for Accessing the Genetic and Functional Diversity of Uncultured Microorganisms. Applied and Environmental Microbiology, 66(6), 2541–2547. http://doi.org/10.1128/AEM.66.6.2541-2547.2000