Streptococcus pneumoniae: Meningitis: Difference between revisions
SullivanMark (talk | contribs) |
No edit summary |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==<i>Streptococcus pneumoniae</i>== | ==<i>Streptococcus pneumoniae</i>== | ||
[[File:9996 lores.jpg|thumb|500px|right|Figure 1. Scanning Electron Micrograph of Streptococcus pneumonmiae by R. Facklam, J. Carr, Source: CDC, http://phil.cdc.gov/phil/details.asp.]] | [[File:9996 lores.jpg|thumb|500px|right|Figure 1. Scanning Electron Micrograph of Streptococcus pneumonmiae by R. Facklam, J. Carr, Source: CDC, http://phil.cdc.gov/phil/details.asp.]] | ||
| Line 109: | Line 110: | ||
==Prevention Methods: Vaccination and Antibiotic Regulation== | ==Prevention Methods: Vaccination and Antibiotic Regulation== | ||
<br>One method to prevent infection by <i>S. pneumoniae</i> is to use polysaccharide vaccines, and these have been available in the U.S. since 1977. This is currently the best method to prevent pneumococcal infection in people with Sickle Cell Anemia, HIV, and high-risk medical conditions. Of the <i>Streptococcus pneumoniae</i> strains that cause disease, 88 % can be prevented when the 23-valent polysaccharide vaccine is used. However, these 23-valent polysaccharide vaccines (the 23 serotypes most likely to cause invasive disease) are not effective in young children, who are at relatively high risk of infection compared to most of the population. A 7-valent protein polysaccharide conjugate pneumococcal vaccine (Prevnar), which is effective in young children, became available in 2000. The State of Alaska made the pneumococcal conjugate vaccine available in 2001, and this led to a 90% decrease in the rate of pneumococcal disease among Alaska Native children [1].<br> | <br>One method to prevent infection by <i>S. pneumoniae</i> is to use polysaccharide vaccines, and these have been available in the U.S. since 1977. This is currently the best method to prevent pneumococcal infection in people with Sickle Cell Anemia, HIV, and other high-risk medical conditions. Of the <i>Streptococcus pneumoniae</i> strains that cause disease, 88 % can be prevented when the 23-valent polysaccharide vaccine is used. However, these 23-valent polysaccharide vaccines (the 23 serotypes most likely to cause invasive disease) are not effective in young children, who are at relatively high risk of infection compared to most of the population. A 7-valent protein polysaccharide conjugate pneumococcal vaccine (Prevnar), which is effective in young children, became available in 2000. The State of Alaska made the pneumococcal conjugate vaccine available in 2001, and this led to a 90% decrease in the rate of pneumococcal disease among Alaska Native children [1].<br> | ||
<br>Since the nearly global overuse of antibiotics, specifically in developed nations, has led to a staggering, rapid increase in the number of multidrug-resistant bacteria, another technique to prevent further pneumococcal infections is to monitor and potentially regulate the use of antibiotics [1]. There have been multiple studies that have reported that repeated and extensive antibiotic use is correlated with a high number of human carriers of antibiotic-resistant <i>Streptococcus pneumoniae</i> (Hennessy et al. 2002). Looking to the future, the most efficacious method to decrease multidrug-resistant <i>S. pneumoniae</i> and prevent untreatable pneumococcal infections is developing a way to responsibly utilize antibiotic drugs (Hennessy et al. 2002). If the overuse of antibiotics continues unchecked as it has throughout the last several decades, then there is a possibility that multidrug-resistant bacteria will reach an unmanageable number.<br> | <br>Since the nearly global overuse of antibiotics, specifically in developed nations, has led to a staggering, rapid increase in the number of multidrug-resistant bacteria, another technique to prevent further pneumococcal infections is to monitor and potentially regulate the use of antibiotics [1]. There have been multiple studies that have reported that repeated and extensive antibiotic use is correlated with a high number of human carriers of antibiotic-resistant <i>Streptococcus pneumoniae</i> (Hennessy et al. 2002). Looking to the future, the most efficacious method to decrease multidrug-resistant <i>S. pneumoniae</i> and prevent untreatable pneumococcal infections is developing a way to responsibly utilize antibiotic drugs (Hennessy et al. 2002). If the overuse of antibiotics continues unchecked as it has throughout the last several decades, then there is a possibility that multidrug-resistant bacteria will reach an unmanageable number.<br> | ||
| Line 118: | Line 119: | ||
==Conclusion== | ==Conclusion== | ||
<br>Many human beings carry non-pathogenic <i>Streptococcus pneumoniae</i> in their nasopharynx. However, when this bacteria becomes virulent and causes infection, especially in the normally sterile brain and cerebral spinal fluid | <br>Many human beings carry non-pathogenic <i>Streptococcus pneumoniae</i> in their nasopharynx. However, when this bacteria becomes virulent and causes infection, especially in the normally sterile brain and cerebral spinal fluid, it can lead to pneumococcal meningitis, and there can be dire consequences. Meningitis can result in several grave neurological conditions and even death. In fact, the brain damage is thought to be mostly attributable to the host’s inflammatory response to the infection. Typically bacterial meningitis is treated with a cocktail of antibiotics, but recently there has been a surge in the number of multidrug-resistant <i>S. pneumoniae</i>, arising due to overuse of antibiotics. These resistant strains of pneumococcus are much harder to treat. Therefore, antibiotic use must be monitored and better inhibitors of host inflammatory systems must be developed in order to make pneumococcal meningitis more manageable and more treatable.<br> | ||
==References== | ==References== | ||
Latest revision as of 14:35, 23 July 2011
Streptococcus pneumoniae
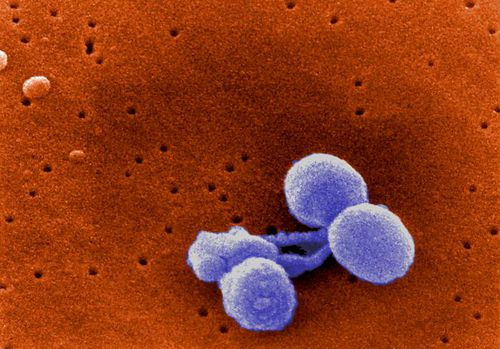
Streptococcus pneumoniae is a non-motile, non-spore forming, gram-positive bacteria of the firmicute phylum. S. pneumoniae is also commonly referred to as pneumococcus [1,2]. Streptococcus pneumoniae are in the shape of a slightly pointed coccus, as seen in Figure 1. The diameter of each single pneumococcus organism ranges from 0.5 to 1.25 micrometers. S. pneumoniae is found singly and in short chains, but mainly these bacteria are found in pairs (diplococci) [2] (Fig. 2). Streptococcus pneumoniae uses fermentation to produce energy via converting glucose into lactic acid. Furthermore, Streptococcus pneumoniae utilizes extracellular enzyme systems in order to obtain carbon and nitrogen, and these processes damage host tissue and permit S. pneumoniae to colonize the host (Todar 2003). Streptococcus pneumoniae and Haemophilus influenzae contribute to the same mucosal microenvironment. Both bacteria are capable of living on their own, but Lysenko et al. (2005) showed that Haemophilus influenzae swiftly outcompetes Streptococcus pneumoniae when they are in the same environment. Thus, H. influenzae can remove populations of the S. pneumoniae pathogen (Lysenko et al. 2005).

Streptococcus pneumoniae exclusively inhabit human beings and are mesophillic, meaning they optimally inhabit areas with temperatures ranging from 30 to 35 degrees Celsius. Streptococcus pneumoniae are most commonly found in the human upper respiratory tract, specifically in the nasopharynx (the nasal passages). Most people carry these bacteria in their nasopharynx, and the harboring of S. pneumoniae within a human is called carriage. Even though most people carry these bacteria in their nasopharynx, most of the time S. pneumoniae does not cause disease [1,2].
A polysaccharide capsule encloses the entire S. pneumoniae cell, and this sugar capsule is key to this bacteria’s virulence. There are hundreds of surface proteins on Streptococcus pneumoniae. Choline-binding proteins are an important group of surface proteins expressed by S. pneumoniae, and this group of proteins helps attach the bacteria to human cells (Todar 2003).
Historical Significance
Louis Pasteur was the first person to isolate Streptococcus pneumoniae, although at the time these bacteria were only known as pneumococcus because they could cause pneumonia in humans. In 1974, this species of bacteria was officially named Streptococcus pneumoniae because it can form chains in liquid.
Streptococcus pneumoniae was a key bacteria species in the development of the field of molecular genetics. Fred Griffith, in 1928, used S. pneumoniae to discover the transformation process in bacteria (Lederberg et al. 2005). In 1944, building off of Griffith’s findings, Avery, MacLeod, and McCarthy demonstrated that DNA and not protein was the genetic material, using the transformative factor in the Streptococcus pneumoniae (Lederberg et al. 2005).
Transmission
Bacterial infection via S. pneumoniae spreads from person to person by means of respiratory droplets. Transmission typically occurs when a carrier coughs or sneezes within six feet of other people, potentially infecting them. Carriers are normally healthy, however, these carriers have the potential to be a source of infection for others [1].
Infection and Spread
Although S. pneumoniae can be harmless at low cell densities, when the concentration of this bacteria becomes too high in its host it can cause infection [2]. Streptococcus pneumoniae can radiate from the nasopharynx to other parts of the body and cause infection and disease there. Streptococcus pneumoniae can cause a wide range of illnesses, including: otitis media (ear infection), sinusitis (sinus infection), bacteraemia (blood infection), pneumonia (lung infection), arthritis, and peritonitis (peritoneum infection, thin tissue lining the walls of the abdomen). Furthermore, S. pneumoniae can infect the lining of the brain and spinal cord, which are normally bacteria-free. Infection in this sterile area leads to meningitis. When Streptococcus pneumoniae infects these sterile areas it is then referred to as ‘invasive’ pneumococcal disease, which can result in serious complications or death [1].
Until 2000, Streptococcus pneumoniae infections caused 100,000 – 135,000 hospitalizations due to pneumonia, 6 million cases of otitis media (ear), and 3300 cases of meningitis in the United States. Since the introduction of the conjugate vaccine in 2001, the incidence of sterile infections has decreased from 21-33 cases per 100,000 population to 13 cases per 100,000 population in the United States.
Moreover, recurrent otitis can lead to hearing impairment. Fourteen percent of people hospitalized for invasive pneumococcal infection of sterile areas, such as meningitis, result in death. Also, meningitis can lead to learning disabilities and possible neurological sequelae. Elderly, young children (< 2 years old), African-Americans, American Indians, Alaska Natives, children who attend day care centers, and people with medical conditions such as HIV infections or sickle-cell disease are all at high risk for infection. There have been outbreaks in institutional settings and in childcare centers [3].
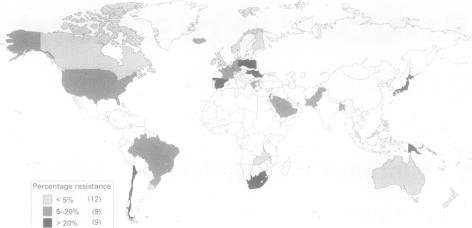
In the United States, the number of strains resistant to multiple classes of drugs is increasing. Furthermore, there has been global spread of the drug-resistant pneumococcus, as seen in Figure 3. Several states in the United States of America have commanded the reporting of drug-resistance Streptococcus pneumoniae and all invasive disease in children, and several American states carry out population-based surveillance. Additionally, a system tracks invasive diseases that arise in children (< 5 years old) that have been vaccinated. The number of young children and young adults infected by S. pneumoniae has been decreasing due to better HIV treatment and the implementation of the conjugate vaccine for children [3].
Meningitis
Symptoms
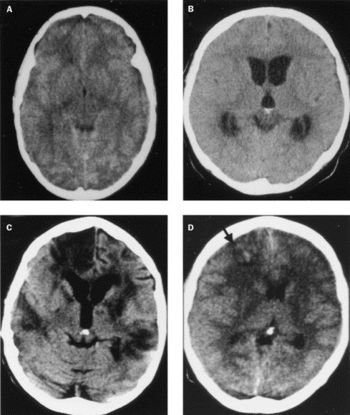
Meningitis can be caused by an invasive pneumococcal infection of the brain and spine. There are many symptoms associated with this disease, and the bacterial meningitis caused by Streptococcus pneumoniae can result in severe symptoms. The signs and symptoms of meningitis normally develop 3-7 days post-exposure. The early signs of meningitis can resemble the symptoms of influenza. For adults, symptoms can include: fever, distinct and severe headache, stiff neck, nausea, vomiting, photophobia (light sensitivity), lack of interest in eating or drinking, seizures, sleepiness, or an altered mental status (confusion). For infants, symptoms can include: constant crying, excessive sleepiness and irritability, inactivity, poor feeding, high fever, stiffness in the baby’s body or neck, seizures, or a bulge in the soft spot on top of a baby’s head (fontanel). A late result of bacterial meningitis can include going into a coma [5,7]. Furthermore, meningitis can cause serious damage to the brain (Fig. 4).
Diagnosis
When an adult or infant is apparently experiencing some of the hallmark symptoms of meningitis, such as neck stiffness, high fever, or severe headache, then that person should be taken to a doctor, who would be able to diagnose the problem. Early diagnosis of meningitis and subsequent early cerebrospinal fluid sterilization with antibiotics are the best methods to combat the possible deleterious effects of pneumococcal meningitis [4,6].
The definitive method of diagnosing meningitis is to obtain a blood sample and a sample of the person’s cerebrospinal fluid, and have these samples analyzed. In order to obtain a cerebrospinal fluid sample, a doctor will perform a spinal tap, a.k.a. a lumbar puncture. This analysis will tell the physician whether the patient has meningitis and also may be able to show the doctor the specific bacterium that is causing the ailment. Identifying the specific cause of the meningitis is of utmost importance, because the treatment and the severity of the illness vary depending on the causative microbe [4,6].
Meningitis: Neuropathology
Streptococcus pneumoniae cause bacterial (also know as pneumococcal) meningitis, which for a long time was considered to be a strictly fatal disease. Due to advances with antibiotic research, penicillin specifically, the mortality levels of meningitis have decreased, but Schuchat et al. (1997) declared that these mortality levels are still too high. In fact, Streptococcus pneumoniae presents the greatest risk of death with bacterial meningitis (Grimwood et al. 2002). Furthermore, fatality is not the only major devastating result from pneumococcal meningitis, because nearly half of the survivors of the disease have been reported to have neurological and neuropsychological sequelae (Grimwood et al. 2002). Many of the deleterious effects of meningitis are caused by the host defense mechanisms, such as inflammatory reaction (Pfister et al. 1997). Therefore, rather than most of the damage being inflicted by Streptococcus pneumoniae, the host is injuring itself in an attempt to stop the infection. So, mediating the host’s defense mechanisms may be the key to limiting the detrimental effects of meningitis (Koedel et al. 2002).
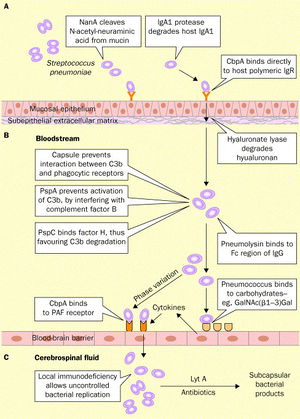
The infection of the brain and spinal fluid by Streptococcus pneumoniae is both difficult and complicated due to the complex defenses that protect the brain, including the blood-brain barrier (Koedel et al. 2002). The process is demonstrated in Figure 5.
The first step in the process of initiating pneumococcal meningitis is S. pneumoniae must adhere to the mucosal epithelium of the nasopharynx. The bacteria that cause meningitis make possible the interaction with the host cell via their own bacterial surface proteins (Koedel et al. 2002). Wisemann et al. (2001) stated that Streptococcus pneumoniae possess over 500 surface proteins, which are used to attach to the nasopharynx mucosal epithelium. Epithelial cells express carbohydrates, and Andersson et al. (1983) found that Streptococcus pneumoniae binds to these surface sugars, specifically GlcNAc(β1→3)Galβ, in order to adhere to pharyngeal epithelial cells.
As mentioned above in the Introduction, the most important protein on the S. pneumoniae cell membrane surface may be the phosphorylcholine, because they are an important pneumococcal adherence factor. Of these phosphorylcholines, there is a group with proteins that attach to it, called choline binding proteins (or the Cbp’s). The most abundant choline binding protein is Cbp A, which is a critical element in pneumococcal adherence (Koedel et al. 2002).
The human body has cells capable of combating pneumococcal infection while the bacteria is adhering to and colonizing epithelial calls. The antibody IgA can uptake and destroy S. pneumoniae by means of phagocytosis (Janoff et al. 1999). However, Streptococcus pneumoniae express a protein called IgA1 protease, which allows the bacteria to cleave and inactivate the antibody and escape phagocytosis (Koedel et al. 2002).
Once the bacteria have adhered to the epithelial cells, the Streptococcus pneumoniae must then invade the mucous and move into the bloodstream, which is termed bacteraemic spread (Koedel et al. 2002). The next challenge facing the pneumococcal invasion is surviving in the blood stream. As stated in the introduction, Streptococcus pneumoniae have a capsular polysaccharide, which has strong antiphagocytic properties, surrounding the entire organism, and this sugar covering is considered integral for the survival of S. pneumoniae in the bloodstream. Austrian (1981) found that all of the Streptococcus pneumoniae isolates that were taken from people with pneumococcal infections had the capsular polysaccharide surrounding them.
The next step in the pneumococcal infection is to cross the blood-brain barrier and infect the normally sterile central nervous system. Tuomanen (1996) stated that infection of the normally sterile bloodstream (bacteraemia) is necessary for Streptococcus pneumoniae, but other events and processes must take place as well. For example, the blood-brain barrier separates the central nervous system and the bloodstream, and the blood-brain barrier prevents not only pathogens from transporting across it, but also prevents the non-specific transport of ions, proteins, cells, and pathogens into the central nervous system (Gloor et al. 2001). Clearly, bacteria attempting to penetrate the blood-brain barrier and enter the central nervous system face a daunting task. Koedel et al. (2002) stated that the mechanism and exact point of entry is not fully known nor understood for most pathogens that cause meningitis. However, Koedel et al. (2002) further reported that several studies now propose that Streptococcus pneumoniae may enter primarily into the central nervous system through the brain endothelium. In order to move through the blood-brain barrier, S. pneumoniae have to adhere to the surface of the brain endothelial cells, attaching to the carbohydrates on the surface of the endothelial cells (Ring et al. 1998, Cundell et al. 1994). Streptococcus pneumonia activates the endothelial cells, causing the endothelial cells, which increases their production of surface-expressed platelet-activating factor (PAF) receptor, which binds the phosphorylcholine that the S. pneumoniae express on their cell walls (Cundell et al. 1995). PAF receptors are quickly internalized once these receptors interact with a ligand, leading researchers to conclude that Streptococcus pneumoniae infest the endothelial cells in vacuoles along with the PAF receptors (Koedel et al. 2002).
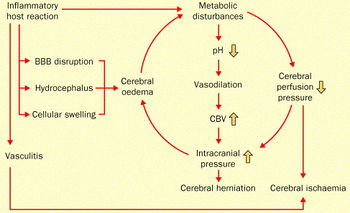
Streptococcus pneumoniae induce the inflammatory defenses of the host once inside the central nervous system (Fig. 6). Autolysis is one pneumococcal process that can initiate host defense. Autolysis involves the bacteria digesting itself by means of autolysins, which are peptidoglycan hydrolases that break down their own cell wall (Lewis et al. 2000). Host immune activation during acute meningitis can occur as a result of interaction with the DNA released from S. pneumoniae upon autolysis. Additionally, products from pneumococcal cell walls can trigger an inflammatory response in a host (Koedel et al. 2002).
The brain damage that occurs during meningitis is mostly attributable to the side effects of the host’s own inflammatory response. When leucocytes are activated by Streptococcus pneumoniae they release proteolytic enzymes and reactive oxygen species, and both of these can potentially damage host tissue (Nussler et al. 1999). Matrix metalloproteinases (MMPs) are one of the proteolytic enzymes released by the leucocytes, and MMPs have been found to disrupt the blood-brain barrier (Lukes et al. 1999). Radical oxygen species produced by leucocytes may contribute to brain damage incurred during meningitis, and this can occur via radical oxygen species attacking polyunsaturated fatty acids, which can damage cell membranes and lead to loss of membrane function (O’Donnell et al. 2001).
Koebel et al. (2002) stated that loss of function in brain endothelial cells could result in loss of cerebrovascular autoregulation, loss of carbon dioxide reactivity of cerebral vessels, and loss of blood-brain barrier integrity. These can all lead to severe brain damage. The most effective methods of preventing brain damage in addition to sterilization of the central nervous system will be to control and interfere with the inflammatory pathways that lead to brain damage during pneumococcal meningitis infections (Koebel et al. 2002).
Pneumococcal Meningitis Infections: Post-Cochlear Implants (Wei et al. 2006)
Compared to the number of normal people at similar ages, individuals who receive cochlear implants have a significantly higher rate of meningitis infections post-implant [35]. Furthermore, the majority of post-cochlear implant meningitis has been caused by Streptococcus pneumoniae. There are risk factors that put people at higher chance of getting meningitis: inner-ear malformations and the presence of cerebral spinal fluid post-implantation. However, Wei et al. (2006) set out to determine whether patients with no risk factors would be at higher risk of pneumococcal meningitis than the average person [35].
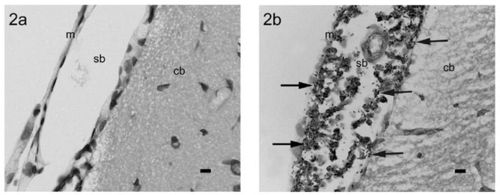
In order to test their hypothesis, Wei et al. (2006) decided to develop a new animal model that would allow Wei et al. (2006) to study the pathogenesis of Streptococcus pneumoniae in animals with and without cochlear implants. Wei et al. (2006) decided to use rats as their model species. Wei et al. (2006) made a control group of 18 normal rats and inoculated them with Streptococcus pneumoniae. Also, they inoculated 6 rats that had cochlear implantations with Streptococcus pneumoniae. Then, they examined the rats for evidence of Streptococcus pneumoniae infection and pneumococcal meningitis. Figure 7 illustrates an example of an infected rat and a rat without an infection.
Wei et al. (2006) found that all of the rats, both the control groups and the group with cochlear implants, indeed did get infected by Streptococcus pneumoniae and subsequently had pneumococcal meningitis. Furthermore, between all of the rats with cochlear implants, all of the routes for infection were present: inner ear, middle ear, and intraperitoneal. The fact that all of the implanted rats had meningitis and all of the routes to infection were displayed provides strong evidence that rats are a good model for studying the association between pneumococcal meningitis and cochlear implants [35].
Antibiotic-Resistant Streptococcus pneumoniae
Penicillin became available in the 1940’s, and physicians treated pneumococcal infections successfully with this antibiotic [1]. However, in the 1960’s, antibiotic-resistant Streptococcus pneumoniae were found in human beings (Hansman et al. 1974). By the late 1970’s, many Streptococcus pneumoniae were found to be resistant to additional types of antibiotics (Jacobs et al. 1978). Today, there are S. pneumoniae found all across the planet that are resistant to multiple drugs and antibiotics (Hennessy et al. 2002).
The spread of multidrug-resistant Streptococcus pneumoniae sharply increased during the 1990 decade. These antibiotic-resistant forms of S. pneumoniae are especially difficult and expensive to treat. For example, there have been many treatment failures due to lack of an effective cure, leading to the death of many human patients. Also, multidrug-resistant Streptococcus pneumoniae necessitate higher doses of antibiotics, longer periods of hospitalization, longer treatment intervals, and more expensive medications. Moreover, these antibiotic-resistant bacteria may also require drugs with additional and more severe side effects [1]. Widespread overuse of antibiotics is the main contributor to the ever-increasing number of emerging drug-resistant bacteria, especially overuse of penicillin to treat Streptococcus pneumoniae [3].
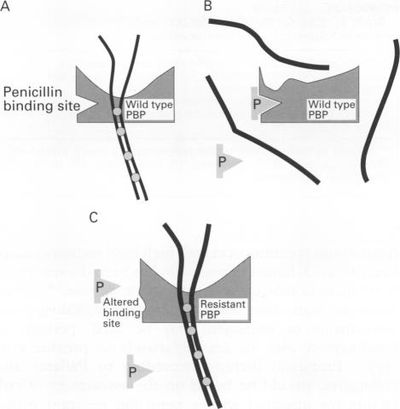
In order for Streptococcus pneumoniae to become antibiotic-resistant, mutant strains must arise. Specifically strains that have mutations on one or more genes that express the penicillin-binding proteins (Fig. 8). Normally, mutations to the transpeptidase-penicillin-binding domain of the penicillin-binding proteins are they types of mutations that can lead to antibiotic-resistance, and this is because penicillin is then unable to bind S. pneumoniae and thus unable to negatively affect the bacteria (Koebel et al. 2002). Furthermore, in order to obtain a high-level of resistance to penicillin, Streptococcus pneumoniae have to acquire several mutations in the transpeptidase-penicillin-binding domain, so as to significantly affect the proteins function (Charpentier et al. 2000). The altered genes that code for the mutated penicillin-binding proteins are sometimes referred to as “mosaic” genes. These “mosaic” genes contain not only native Streptococcus pneumoniae DNA, but also native DNA is mixed with foreign DNA. This foreign DNA was probably taken up from DNA fragments originating from antibiotic-resistant bacteria. The foreign DNA can be taken up and inserted into the chromosome of the Streptococcus pneumoniae (horizontal gene transfer) (Koebel et al. 2002).
Prevention Methods: Vaccination and Antibiotic Regulation
One method to prevent infection by S. pneumoniae is to use polysaccharide vaccines, and these have been available in the U.S. since 1977. This is currently the best method to prevent pneumococcal infection in people with Sickle Cell Anemia, HIV, and other high-risk medical conditions. Of the Streptococcus pneumoniae strains that cause disease, 88 % can be prevented when the 23-valent polysaccharide vaccine is used. However, these 23-valent polysaccharide vaccines (the 23 serotypes most likely to cause invasive disease) are not effective in young children, who are at relatively high risk of infection compared to most of the population. A 7-valent protein polysaccharide conjugate pneumococcal vaccine (Prevnar), which is effective in young children, became available in 2000. The State of Alaska made the pneumococcal conjugate vaccine available in 2001, and this led to a 90% decrease in the rate of pneumococcal disease among Alaska Native children [1].
Since the nearly global overuse of antibiotics, specifically in developed nations, has led to a staggering, rapid increase in the number of multidrug-resistant bacteria, another technique to prevent further pneumococcal infections is to monitor and potentially regulate the use of antibiotics [1]. There have been multiple studies that have reported that repeated and extensive antibiotic use is correlated with a high number of human carriers of antibiotic-resistant Streptococcus pneumoniae (Hennessy et al. 2002). Looking to the future, the most efficacious method to decrease multidrug-resistant S. pneumoniae and prevent untreatable pneumococcal infections is developing a way to responsibly utilize antibiotic drugs (Hennessy et al. 2002). If the overuse of antibiotics continues unchecked as it has throughout the last several decades, then there is a possibility that multidrug-resistant bacteria will reach an unmanageable number.
Supplies of the pneumococcal conjugate vaccine are inadequate, and the 23-valent polysaccharide vaccine is underused [3]. In order to prevent infections in adults and children, the use of both the 23-valent polysaccharide vaccine and the pneumococcal conjugate vaccine respectively must undergo expanded implementation. Furthermore, the increase of vaccination may lead to a decrease in drug-resistant pneumococcal carriage. If less people get infected because they got vaccinated, then the use of antibiotics may decrease, which in turn could put a stop to the increasing number of antibiotic-resistant bacteria.
Conclusion
Many human beings carry non-pathogenic Streptococcus pneumoniae in their nasopharynx. However, when this bacteria becomes virulent and causes infection, especially in the normally sterile brain and cerebral spinal fluid, it can lead to pneumococcal meningitis, and there can be dire consequences. Meningitis can result in several grave neurological conditions and even death. In fact, the brain damage is thought to be mostly attributable to the host’s inflammatory response to the infection. Typically bacterial meningitis is treated with a cocktail of antibiotics, but recently there has been a surge in the number of multidrug-resistant S. pneumoniae, arising due to overuse of antibiotics. These resistant strains of pneumococcus are much harder to treat. Therefore, antibiotic use must be monitored and better inhibitors of host inflammatory systems must be developed in order to make pneumococcal meningitis more manageable and more treatable.
References
Websites:
1. CDC Streptococcus pneumoniae Research
2. page for Streptococcus pneumoniae
3. CDC Disease Information, Streptococcus pneumoniae
4. Mayo Clinic Meningitis Diagnosis
5. Mayo Clinic Meningitis Symptoms
Research Articles:
12.Davidson M, Parkinson AJ, Bulkow LR et al. The epidemiology of invasive pneumococcal disease in Alaska, 1986-1990 -ethnic differences and opportunities for prevention. J Infect Dis. 1994; 170:368-76.
14.Rudolph, KM, Parkinson AJ, Reasonover AL, Bulkow LR, Parks DJ Butler JC. 2000. Serotype distribution and antimicrobial resistance patterns of invasive isolates of S. pneumoniae: Alaska 1991-8. J Infect Dis 490-6.
31.Lewis, K. 2000. Programmed death in bacteria, Microbiol Mol Biol Rev 64 (2000), pp. 503–514.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.
