The Burden of C. difficile and its Link to Nosocomial Infections
Introduction
Clostridium difficile infections are the leading cause of hospital-associated infections (HAIs), or nosocomial infections, in the United States. The infections are becoming increasingly serious, and the presence of hypervirulent strains are causing epidemics estimated to cost the USA healthcare system approximately $3.2 billion annually (22). This breaks down to $3,006-$15,397 per episode (23). These new strains are resistant to fluoroquinolones, and produce more spores and toxins than what has been studied historically (22). It is important to study this bacteria, as it is becoming an increasing risk to public health. The Center of Disease Control is working to prevent C. difficile infections by incorporating its State Antibiotic Resistance Programs across the country, with a goal of reducing the number of incidences of infection by half (26).
Types of HAI
Central line-associated bloodstream infections (CLABSI)
Vascular catheters are used frequently in the United States for a variety of medical purposes, including the administration of medicine, nutritional fluids, or conducting medical tests. They are especially used in intensive care units (ICUs) as central vein catheters are in closer contact to the heart compared to the familiar IV (5). However, these catheters present a risk of bloodstream infection; bacteria or viruses may colonize on the internal surface of the catheter where fluids are being transported, or on the external surface of the catheter. These are serious infections, and symptoms may include a fever, chills, or sore, red skin around the site of infection (3).
Catheter-associated urinary tract infection
Urinary catheters are tubes inserted through the urethra into the bladder to drain urine, and are received by 15-25% of hospital patients (6). When bacteria are present on the catheter, at the time of insertion, or on the skin surrounding the insertion site, a urinary tract infection (UTI) may occur. Signs of infection include lower abdomen pain, fever, or burning sensation while urinating. Risk of infection is increased the longer the catheter is in place, as bacterial biofilms may grow and eventually colonize the patient’s skin, tissue, or organs involved in the urinary tract (8).
Surgical site infections (SSI) Surgical site infections are infections that develop at the site of recent surgery. It is estimated that 1 to 3 out of every 100 patients undergoing surgery develop an SSI (2). These infections range in intensity and can be caused by a variety of bacteria. SSIs may occur topically on the skin surrounding the surgery site, in the muscle or tissue beneath and surrounding the surgery site, or in organs or implant material. The infections occur due bacteria such as staphylococcus, streptococcus, and pseudomonas that come in contact to the surgery wound. This may occur via contaminated surgical instruments, contact from the doctor or caregiver, from microbes already present on the patient’s skin, or from airborne microbes. Factors that increase the risk of SSIs include surgeries lasting over two hours, old age, smoking, and any pre-existing diseases or reasons for a compromised immune system (1). Signs of infection include fever, pain and swelling around the area of the surgery, and drainage of cloudy fluid (pus) from the surgery wound. Pus can be cultured to identify the types of bacteria causing infection, and therefore identify the best treatment option. These infections may require patients to remain in the hospital for as long as seven extra days (1).
Ventilator-associated pneumonia
Ventilators are used to deliver oxygen to a patient, using a tube through the patient’s mouth, nose, or hole in the front of the neck. Diseases, most commonly being ventilator-associated pneumonia (VAP), can occur when bacteria enter the lungs and lower respiratory tract. This may be due to biofilms forming on the tube connected to the ventilator, in the patient’s neck and lungs, or via air transmission at the site of tube insertion. Unfortunately, this is extremely common because patients requiring ventilators are often immunocompromised and have underlying medical conditions. Most of the causative bacteria are gram-negative bacteria, however gram-positive cocci also contribute significantly to VAP (10).
Clostridium difficile as a Leading Cause
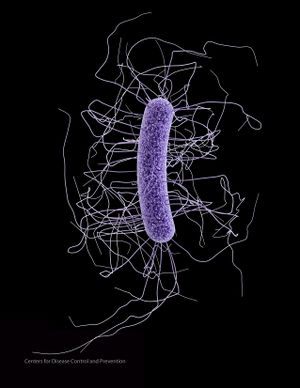
C. difficile is a gram-positive firmicute that is found in a wide variety of environments including water, air, feces, and soil (Figure 1). It is anaerobic, can form endospores that may survive for up to two years when conditions are unfavorable, and produces endotoxins (10). It has been found that C. difficile prioritizes growth over toxin production, therefore it is able to grow and colonize, then release toxins in a larger quantity (10). Infection by C. difficile involves a range of physical symptoms, including mild to severe diarrhea, toxic megacolon (infection and inflammation of the colon), and can even cause death in extreme cases. Specifically, about 5-10% of cases end in mortality, averaging to an estimated 14,000-20,000 deaths annually in the US. C difficile is extremely prevalent in the US; however, it is also found worldwide (15).
There are many reasons why C. difficile is the most common bacteria in nosocomial infections. Inherently, its optimal growth temperature is that of the human body, therefore allowing it to thrive in a human host. It is unaffected by many antibiotics, therefore when a patient takes an antibiotic, the healthy gut flora die and C. difficile no longer has competition for nutrients or space. Also, the genome of C. difficile has been sequenced, and researchers found that a large region of the genome codes for mobile genetic machinery such as transposons used in conjugation. This increases the rate of successful gene incorporation into the host cell’s genetic material (10).
Furthermore, much of C. difficile’s virulence is regulated by its environmental conditions. For example, surface protein expression is upregulated in times of stress, allowing it to stick to its host cells more strongly. It is also able to upregulate toxin production when temperature increases; this adaptation allows the bacteria to reduce energy expenditure by only producing toxins when in contact with host cells (10). In hospitals
In a study looking at C. difficile’s presence as a hospital-acquired infection, it was found that the most commonly contaminated surfaces in the hospital included the bathroom handrails, toilet seats, and tray tables (11). This is expected, as C. difficile are found in human feces and therefore in bathrooms. They are transmitted often in spore form on the hands of doctors and other medical personnel (12). The most common way to diagnose a patient with a C. difficile infection (CDI) is to take a stool sample and test for the presence of the bacteria or its toxins. Hospitals and labs that test for C. difficile must report to Infection Prevention and Control daily (23).
C. difficile is similar to other prevalent hospital pathogens, such as MRSA and vancomycin-resistant enterococci. They all inhabit the skin and are transmitted between healthcare providers and patients. However, C. difficile is distinct in that it forms spores, which are resistant to alcohols and common hospital cleaning agents. Also, C. difficile has not been shown to be affected significantly by hand washing or use of alcohol hygiene products, whereas these alcohol products are very effective against MRSA and other organisms that do not form spores (23).
In nursing homes
Especially vulnerable to C. difficile infections are those in nursing homes. Many people living in nursing homes are attempting to live a healthy lifestyle and not require hospitalization; however, pathogens such as C. difficile can cause severe complications in elderly people, many of whom are immunocompromised due to old age or a preexisting medical condition. Infections can cause malnutrition, physical weakness and frailty, or possible hospitalization for elderly individuals. Over 80% of C. difficile associated deaths are in Americans older than 65 years, and more than 100,000 infections occur annually in this age bracket of nursing home residents (26).
Some regions may experience high transmission rate between hospitals and the nursing home. Holding nursing homes to similar standard as hospitals, in regards to sanitation measures and reporting infection rates, can help decrease the presence of C. difficile. There are currently efforts to create prevention programs, with goals such as allocating more money to education and prevention of C. difficile infections, reducing hospital transmission of infections, and designing reports and standards that nursing homes must follow when diseases and infections are diagnosed. Proposals have also been made to penalize hospitals for excess C. difficile infections, as incentive to reduce the infection prevalence (25).
Toxin Production
Include some current research in each topic, with at least one figure showing data.
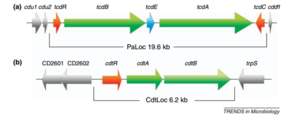
Strains and Identification
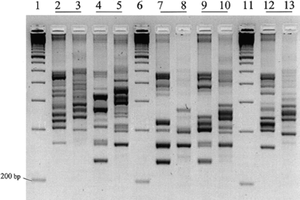
Include some current research in each topic, with at least one figure showing data.
Treatment
Include some current research in each topic, with at least one figure showing data.
Prevention
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
