The Development of Pathogenesis in the Zika Virus
By: Jack Crow

General Information
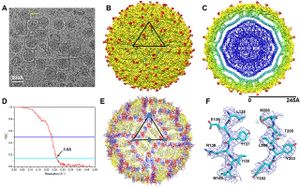
Zika virus (ZIKV) is a mosquito-borne single stranded, positive sense RNA virus, member of the family Flaviviridae and member of the genus Flavivirus. [2] The main vectors, or carriers, of the virus are Aedes mosquitoes, for instance A. aegypti. [2] Zika virus is related to other Flaviviruses: dengue, yellow fever, Japanese encephalitis, and West Nile viruses. [3] The virus causes causes a spectrum of effects that range from mild symptoms that may go unnoticed, to fever, macropapular body rash, headache, conjunctivitis, myalgia and joint pain. Furthermore, as of April, 2016, The CDC has established a causal relationship between the virus and microcephaly and other severe brain problems, in infants. Zika infections in adults has also been shown to cause Guillain-Barré syndrome. [2] Although the virus was first isolated nearly seventy years ago, less than twenty human cases had been reported prior to 2007; since 2007 there have been an estimated one million Zika virus infections, most notably in Brazil [4] There is currently no vaccine against the Zika virus, and extremely limited treatment options for Zika derived microcephaly. [5]
Origin, Mutation and Spread of the Virus
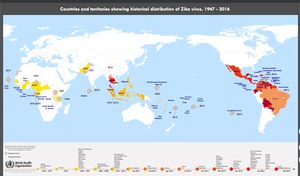
The virus was first isolated from a rhesus monkey in the Zika Forest of Uganda, in 1947.[6] It was first isolated in human hosts in Nigeria in 1968.[6] It is believed the virus spread from Africa to Asia (Pakistan and Malaysia) as early as 1977. [7] In 2007 physicians identified the outbreak of illness in the on the Yap Island in the Federated States of Micronesia. Although the patients tested positive for Dengue virus infection using an IgM test, samples sent to the CDC were found to contain genomes corresponding to the Zika virus RNA genome.[8] From Micronesia the virus is believed to have spread to Brazil sometime in 2014, and north through Central America to Mexico in 2015-2016.[8]
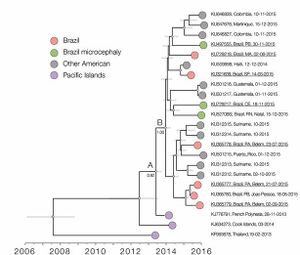
Faria et al (2016) discuss three hypotheses for the introduction of the virus to Brazil: during the 2014 World Cup soccer tournament (June and July, 2014), during the Va’a canoe race held in Rio de Janeiro (August, 2014) and during the 2013 Confederations Cup soccer tournament (June, 2013). The investigators state that their molecular clock analyses suggest that the introduction of the virus to Brazil predates the World Cup and Va’a canoe race, but that the Confederations Cup ended before the symptoms associated with the more pathogenic serotypes were reported in French Polynesia. Although it is difficult to categorize new serotypes of the virus, there are believed to be two major lineages of the virus, four major genotypes and many individual isolates of the virus. Faria et al. (2016) estimate that American serotypes of the Zika virus are mutating at a rate from 0.98 to 0.00106 nucleotide substitutions / site / year. [9] They suggest this mutation rate is consistent with mutation rates of previous epidemics, and is higher than mutation rates in other flaviviruses. Consequently, it is likely that the American serotypes of the Zika virus are currently hypermutable, but may decrease in mutability in the coming years. [9]
Researchers have suggested that if there is a link between microcephaly and the Zika virus genome, the link arose somewhere in the lineages ancestral to the French Polynesian outbreak. [9] Faria et al. (2016) identified eleven amino acid mutations between the two major lineages of the Zika virus. These researchers also mapped the predicted physiological change due to the amino acid substitution in seven of the eleven mutations. They suggest that the only mutation that would likely cause a change in the characteristics of the protein, is the mutation on the helicase protein (Y2086H) that may increase hydrophobicity in the region of the mutation. [9] If there is indeed a relationship between certain lineages of the Zika virus and microcephaly, exogenous factors may play a larger role in the development of microcephaly than the genetic makeup of the Zika virus. The lack of major physiological differences between the microcephaly inducing Zika lineages and non-microcephaly inducing lineages supports this theory. However, more work is necessary to elucidate the role of mutations in increased pathogenesis and microcephaly inducing Zika lineages.
Transmission Biology
The flaviviruses are transmitted to humans by mosquitoes, Zika is transmitted by Aedes family mosquitoes. Upon blood feeding, the virus enters, and passes through, the epidermal, dermal and Langerhans cells of the skin. Skin firbroblast cells and epidermal keratinocyte cells are permissive to the virus. Similar to the Dengue virus, the Zika virus initiates apoptosis of epidermal cells, subverting immune responses such as autophagy, and releasing high number of viral particles. The envelope proteins of viral particles interact with receptor proteins of host cells, to initiate host cell - virus receptor mediated endocytosis.[11] The functional and physiological interaction of envelope proteins and host cell receptor proteins is not well understood. It is believed the virus migrates from skin cells to regional lymph nodes and into the bloodstream. From these cells, the virus may be transported to organs and tissues including: the central nervous system, the skeletal muscles, myocardium, and perhaps transplacentally to the foetus.” [4] There is also mounting evidence that the Zika virus can be sexually transmitted. In 2013, during the French Polynesian outbreak, researchers isolated Zika in a patient's semen. [12] Researchers have also identified several cases of Zika infection in patients without exposure to Zika infected regions, blood transfusions. For instance, physicians in Paris isolated the Zika virus in a patient without previous exposure, who had sexual contact with a Brazilian man [13]
The Zika virus is believed to interact with human immune system similarly to other flavivirus family viruses. The human immune response to flaviviruses is a complicated relationship. These viruses can be both beneficial and pathogenic, and there may be interactions between different viruses and phenotypes of viruses.[5] Building a comprehensive understanding of the disease, and the continuum of possible effects of the disease, is essential in developing a functional characterization of Zika virus infection.
The relationship of the Zika virus with microcephaly, and other severe neurological diseases, is not currently well understood. An increase in the number of infant microcephaly cases was first noted in Brazil in 2015.[14] Researchers have been cautious to associate cases of microcephaly with the virus, as it has been more than half a century since a virus has been linked to significant congenital defects (Rubella virus) and no known flavivirus has been linked with congenital defects, let alone microcephaly.[15] Although there is not any evidence, genetically or otherwise, that directly links Zika to microcephaly, yet, as of April 2016, researchers have established a causal relationship between Zika and microcephaly using Shephard’s criteria for causal relationships.[15] Further complicating the matter of elucidating the relationship between Zika and microcephaly, is the role of other factors in the development of microcephaly, or other severe symptoms. Researchers have hypothesized that co-infection with the Chikungunya virus, previous infection with Dengue, or other flavivirus,[16] and genetic predisposition to the disease may increase the pathogenesis of the virus.[9] Therefore, further research is necessary to determine the extent of Zika virus’s role in microcephaly.
Economic and Societal Impact of the Virus and Microcephaly
Microcephaly and Social Impact
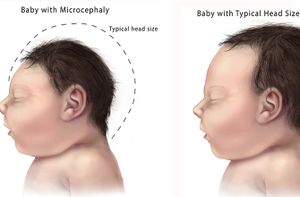
Microcephaly is a medical condition in which the circumference of an infants’ head, is at least two standard deviations below the average circumference for that age and sex. Microcephaly can be caused by a variety of factors including: inheritance of a microcephaly allele, down syndrome, fetal alcohol syndrome and radiation exposure. The disorder is often characterized by moderate to severe intellectual impairment, poor motor function, poor speech and seizures. There have been more than 4,000 reported cases in Brazil since early 2015, [18] and some experts believe that number may rise to as high as 40,000 cases in the next six months.[19]
The disease causes a number of sociological problems. NPR reports that abandonment of mothers of children with microcephaly and children with microcephaly has increased in Brazil since the Zika outbreak.[20] Futhermore, abortion is illegal in many parts of Brazil, creating concerns over criminality, on top of preexisting societal, moral and religious dilemmas, in a country in which 65% of the population identifies as Roman Catholic. Raising a child with severe neurological impairments is difficult in even the most stable households, and poses an enormous burden on single mothers. There is often little funding available for families, or single mothers, raising children with microcephaly. The amount of labor necessary for raising a child with microcephaly, equates, ostensibly, to a full time job. Single mothers often struggle to balance childcare with employment, and many children with microcephaly ultimately end up in facilities run by the state.
Microcephaly and Economic Impact
Beyond the personal suffering caused by the virus and microcephaly, there are many potential economic ramifications as well. There are currently no restrictions on travel to Brazil, or endemic regions, because of the Zika virus. Yet, there may be potential for negative impact on the tourism industry in endemic regions. The comparable outbreaks of Chikungunya and Dengue in Asia, reportadly caused a 4% decline in annual revenues derived from tourism.[18] Brazil invested $350,000,000 in Dengue control and research efforts in 2015, and it is likely this funding will increase to incorporate Zika research in the coming years.[18] Decline in foreign investment is another major concern. Costenala et al. (2016), cited a $2.7 billion dollar decline in foreign investment in mainland China, and a 62% decline in Hong Kong, following the SARS outbreak in 2003; this trend was reversed after the outbreak ended.[18]
Intellectual impairment caused by the Zika virus, may prevent affected children from ascending to the intellectual potential they may have otherwise reached. There has not been enough time to judge the effect of Zika derived microcephaly in children’s academic development, but, in general, microcephaly often leads to severe intellectual development, “possibly requiring lifelong intensive care.”[21] Although we may not currently know the extent of damage caused by Zika related microcephaly, research has shown severe retardation of brain growth in fetuses with the Zika virus genome present.[22] If such trends are indeed the standard for Zika related microcephaly, affected children may not be able to attend school. It may be relatively cynical to discuss the long-term effects of children with microcephaly. Regardless, it is likely impaired academic and intellectual development would, consequently, lower economic productivity in endemic regions, as affected children reach adulthood. Furthermore, fear of the effects of the Zika virus, may lead to a decrease in the number of parents willing to have children in endemic regions. This may lead to a decrease in population growth in these regions, further exacerbating a decrease in potential economic productivity.
Diagnosis and Identification
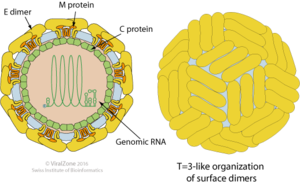
Difficulties arise in developing strong diagnostic standards for Zika identification. Confirmation of virus presence in potential hosts, is typically done in laboratory settings, “by testing serum for viral nucleic acid of virus-specific IgM and IgG antibodies.”[5] Huang et al. (2016) discuss the difficulty in evaluating potential infected hosts more than seven days after the initial acquisition of the virus. After seven days, “negative RT-PCR results… do not rule out flavivirus infection.”[5] Furthermore, Huang et al. (2016) report cross reactivity of antibodies between Zika and Dengue viruses. Diagnostics, and vaccine development, are further complicated by possible interactions between Zika and Chikungunya viruses. Therefore a positive diagnosis of Zika virus infection may not always necessarily confer the severe adverse health effects currently associated with the Zika virus, such as the development of microcephaly.
Diagnosis Case Study
To obtain a correct diagnosis of Zika virus, investigators must rule out a host of different viral, bacterial and other pathogenic disease causing agents. Take for instance, the case of a Canadian woman who was infected with the virus during a trip to Thailand, discussed by Fonesca et al. (2014).[24] On return to Canada, the woman noted the onset of fever and chills, and the development of a papular rash. Blood, nasopharyngeal swab, and urine samples were examined for presence of measles and other disease causing agents. This is typical of the process of elimination analysis of samples for travel-related illnesses. Upon elimination of diagnoses of measles and other traditional diseases acquired abroad, investigators examined hemoglobin, leukocyte, platelet, creatine, electrolyte, alanine aminotransferase and alkaline phosphotase levels. Further investigation of blood smears ruled out malaria, and other bacterial and non-bacterial blood parasites. Consequently, blood samples were analyzed using Dengue virus IgM and IgG serological tests, that resulted positive for Dengue infection. Although the patient’s blood sample tests and symptoms corresponded with an acute Dengue virus infection, later blood analyses showed limited seroconversion of IgM and IgG antibodies relative to earlier blood samples. This prompted the physicians to order a comprehensive analysis of samples, to test for the presence of irregular flavivirus particles. Reverse transcriptase PCR was used to partially sequence pathogen genomes, and revealed the presence of a virus highly homologous to the Asian lineage of the Zika virus. Only upon such extensive examination of potential pathogens, were samples sent to the CDC for confirmation of presence, and full genomic sequencing, of the Zika virus.
The previously described case highlights many of the difficulties associated with obtaining an accurate diagnosis of the Zika virus in human hosts. Numerous tests must be employed to eliminate the possibility of non-Zika pathogens as the disease causing agent. Furthermore, the patient must have access to physicians qualified to make such evaluations, and laboratories equipped to perform such analyses. Furthermore, the symptoms associated with the Zika virus are often subtle enough to evade recognition by the human host. While this may be beneficial for the general infection of healthy individuals, it can facilitate the spread of the virus, without timely recognition by disease monitoring agencies. The limited practical effects of Zika virus in healthy individuals may not put the majority of the population at any significant health risk, compounding factors, such as multiple infections, weakened immune systems, genetic pre-disposition to the detrimental health effects of flavivirus infection and many other potential factors may put certain demographics at risk, especially in regions where the presence of the disease goes unrecognized. The unrecognized spread of Zika may also facilitate the mutation of more virulent serotypes in disparate regions, as current iterations of the virus have been shown to possess a high rate of mutation.
Although there has been a strong effort to combat the spread and severity of health risks associated with the virus, impoverished regions are at greater risk for epidemic spread. Regions, perhaps most significantly in South and Central America, have fewer resources for the identification and treatment of the Zika virus. Countries such as Bolivia, Ecuador, Colombia, El Salvador, Paraguay and other American countries may lack facilities for extensive serological analysis of samples, especially in rural regions. Even if these regions possessed strong health care systems that could identify microcephaly cases, the lack of treatment options pose barriers to any significant reduction in abnormalities associated with Zika.
Control, Prevention and Treatment

Vaccine development is still in the experimental phases of development. Modification of existing flavivirus vaccine may provide the most promising pathway for Zika virus vaccine development. Dengvaxia, the first registered Dengue virus vaccine, is in stage III of clinical drug development, and is approved for use in infected individuals between the ages of nine and forty-five in endemic areas.[26] Furthermore, vaccines against yellow fever and Japanese encephalitis have been available for more than twenty years.[5] However, Huang et al. (2016) caution against over-dependence on existing flavivirus vaccines, citing positive and or negative interference in patients with flavivirus immunity and of vaccine-induced development of Guillain-Barre syndrome.[5]
Efforts to control the spread of the virus, through control and eradication of vectors, have achieved little success. Aedes family mosquitoes have not been impacted by the mass spraying of insecticides in regions endemic with Dengue.[5] Furthermore, there is a lack of knowledge on the relationship between vector mosquitoes and flaviviruses, Zika in particular, and the role of vectors in transmission of the viruses. Epidemiologists have suggested the potential biological and genetic approaches as vector control methods. Examples of potential biological control methods include the use of infection of endogenous mosquito vectors with Wolbachia bacteria. This infection causes non-viable reproduction between a Wolbachia infected mosquito and a non-Wolbachia infected mosquito. Another possibility is the use of irradiation of vectors to produce sterile males. Yet, both possibilities are highly labor and cost intensive practices. Furthermore, both require the alteration of large amounts of vector populations that must out compete healthy endogenous vectors. Genetic alternatives, such as CRISPR gene editing, may provide promising alternatives in modification of essential, or even certain non-essential, genes to limit the efficacy of virus transmission. Unfortunately, there isn’t a solid consensus on the morality of such methods, and, more practically, it is unlikely health organizations are ready to implement CRISPR technology on a scale large enough to significantly impede virus transmission.
The 2016 Rio Summer Olympic and the Safety of Tourism in Regions With Active Zika Transmission
The 2016 Summer Olympics are scheduled to be held from August 5th to August 21st, in Rio de Janeiro and five other cities: São Paulo (Brazil's largest city), Belo Horizonte, Salvador, Brasília (Brazil's capital), and Manaus. Researchers have raised concerns over the potential spread of Zika, due to the influx of foreign tourists for the games. [27] Rio de Janeiro’s 26,000 suspected Zika cases are the highest of any state in in Brazil. Brazilian officials expect Zika infections to drop in the winter months (July to September), following the trend of other Aedes family transmitted viruses such as Dengue. However, this optimistic projection is tempered by an abnormally high Dengue infection rate [28], that alarmingly coincides with the largest attempt at systematic mosquito eradication in Brazil's history. [29]. These concerns are augmented by the link between Zika and sexual transmission, as well as the work Faria et al. (2016) that shows the Brazilian Zika outbreak can be traced to a single infected traveller. [30] While no one can accurately predict the rate at which the virus will continue to spread outwards from Brazil, the estimated 500,000 tourists expected to travel to Brazil for the Summer Olympics is cause for concern. The International Olympic Committee has stated that the Rio organisers will inspect venues and remove stagnant water in the area surrounding the stadiums daily, both before and after the games. [31] However in the interest of safety, all tourists should be aware of the dangers of Zika, especially pregnant mothers.
Preliminary Travel Advisory
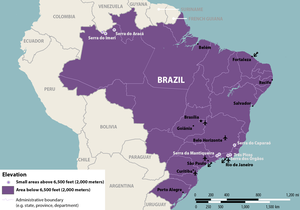
Without further information, it will be difficult for researchers to quantify the likelihood of Zika infection for tourists visiting Brazil for the Summer Olympics. Those intent on visiting Brazil in the coming months should monitor the situation for any developments, and contact health professionals for an assessment of genetic predisposition for Guillain-Barré syndrome and acute disseminated encephalomyelitis. The CDC advises tourists visiting Brazil to protect themselves from mosquito bites, to the greatest extent possible. Furthermore, the CDC notes that Aedes family mosquitoes are generally unable to live at elevations above 6,500 feet (2,000 metres), and travelers who plan on visiting regions above 6,500 feet are at minimal risk for Zika infection. [32] Perhaps most importantly for those that travel to Brazil, or any region in which Zika is currently active, should be aware of the main mechanisms of Zika transmission and the symptoms of Zika infection. The CDC suggests that travelers use condoms, or abstain from sexual intercourse entirely, throughout the duration of any visit to a Zika infected country. The virus often causes only mild, or no, noticeable symptoms. Any traveler who notices fever-like symptoms during, or following, a visit to Brazil, should seek medical attention immediately to identify the presence of the virus, and curb further spread. If possible, any traveler to a region with active Zika transmission, should plan on seeking medical attention upon return arrival. This will ensure the traveler is not carrying the virus, preventing spread and making unknowing carriers aware of the viruses presence in the event that effects associated with carrying the virus over the long term should arise.
Conclusion and Future Outlook
The most promising control efforts may combine some combination of the possibilities previously discussed, along with a systemic enhancement of regional diagnostic and health care capabilities. The paramount goal of any effort to limit the Zika virus, must protect pregnant women and their babies from the serious health risks associated with Zika. If such treatments become available, it is possible that the dangers associated with Zika may be greatly diminished over the long-term. If it is indeed impossible to successfully eradicate vector species, and, or, eliminate the virus from vector species, this type of treatment may afford the best possible outcome, as the virus itself is rarely the direct cause of any serious health problems. To reach such levels of treatment development, it is essential that investigators develop a globally integrated research network focused on elucidating the relationship between Zika virus infection, the extraneous compounding factors and the severe health problems that result. There is promising work underway across the world. Knowledge of the Zika virus was severely limited prior to 2007. But, of 313 papers on Zika, 225 were published in the first four months of 2016. The rapid mobilization of health organizations, and a dramatic increase in public funding has spurred on extensive research projects across the world. Furthermore, the effective response of the scientific community to other flavivirus epidemics such as Dengue, Japanese encephalitis and yellow fever provide promising pathways for the development of effective Zika control methods.
Sequences, Accession Numbers and Supplementary Data
The accession numbers and associated sequences isolated and / or used by Faria et al. (figure 3)[9] can be found at: http://datadryad.org/resource/doi:10.5061/dryad.6kn23
References
- ↑ [1] VectorBase Aedes aegypti organism information. N.D. N.A.
- ↑ 2.0 2.1 2.2 [2] Malone et al. "Zika Virus: Medical Countermeasure Development Challenges" 2016. PLoS Biology http://dx.doi.org/10.1371/journal.pntd.0004530
- ↑ [3] Sikka et al. "The emergence of zika virus as a global health security threat: A review and a consensus statement of the INDUSEM Joint working Group (JWG)". 2016 J Global Infect Dis 2016;8:3-15
- ↑ 4.0 4.1 [4] "Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease". Chan et al. 2016. JOI 72:507-524
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 [5] Haug et al. "The Zika Challenge". 2016. NEJM doi:10.1056/NEJMp1603734
- ↑ 6.0 6.1 [6] "Zika Virus Outside Africa. Emerging Infectious Diseases". Hayes EB 2009;15(9):1347-1350. doi:10.3201/eid1509.090442.
- ↑ http://www.who.int/emergencies/zika-virus/zika-historical-distribution.pdf?ua=1
- ↑ 8.0 8.1 [7] Duffy et al. "Zika Virus Outbreak on Yap Island, Federated States of Micronesia". 2016. N Engl J Med 2009;360:2536-43.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 [8] Faria et al. "Zika virus in the Americas: Early epidemiological and genetic findings". 2016 DOI: 10.1126/science.aaf5036
- ↑ [9] Withnall. 2016 "Countries and territories showing historical distribution of Zika virus, 1947 - 2016". WHO.
- ↑ [10] Mukhopadhyay, Kuhn and Rossmann. "A structural perspective of the flavivirus life cycle". Nature; 01/01/2005;3:13.
- ↑ [11] Musso, Roche, Robin, Nhan, Teissier and Cao-Lormeau. "Potential Sexual Transmission of Zika Virus" 2015. National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC); 02/2015;21:359.
- ↑ [12] Eric D'Ortenzio, Matheron, de Lamballerie, Hubert, Piorkowski, Maquart, et al.. "Evidence of Sexual Transmission of Zika Virus". 2016. NEJM. DOI: 10.1056/NEJMc1604449
- ↑ [13] Kleber de Oliveira, Cortez-Escalante, De Oliveira, et al. "Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy — Brazil, 2015". MMWR Morb Mortal Wkly Rep 2016;65:242–247.
- ↑ 15.0 15.1 [14] Rasmussen et al. "Zika Virus and Birth Defects — Reviewing the Evidence for Causality" 2016. DOI: 10.1056/NEJMsr1604338
- ↑ [15] Fagbami et al. "Heterologous Flavivirus Infection-Enhancing Antibodies in Sera of Nigerians". 1988. Am. J. Trop. Med. Hyg., 38:1. 205-207.
- ↑ [16] N.A. "Zika Virus – Things you must know to protect your family". N.D.
- ↑ 18.0 18.1 18.2 18.3 [17] Costenla, de Broucker and del Campo. "The Potential Economic Impact of the Zika Virus" 2016.
- ↑ [18] Goldman. "Zika's Ground Zero: Brazil hits 4,000 suspected Zika-related cases of birth defects". 2016.
- ↑ [19] Garcia-Navarro. "Moms And Infants Are Abandoned In Brazil Amid Surge In Microcephaly". 2016. All Things Considered
- ↑ [20] "NINDS Microcephaly Information Page" 2016. NIH.
- ↑ [21] Mlakar et al. "Zika Virus Associated with Microcephaly" 2016. NEJM. DOI: 10.1056/NEJMoa1600651
- ↑ [22] Zika virus (strain Mr 766). ViralZone Main Page.
- ↑ [23] Fonseca et al. "First Case of Zika Virus Infection in a Returning Canadian Traveler". The American Journal of Tropical Medicine and Hygiene. 2014;91(5):1035-1038. doi:10.4269/ajtmh.14-0151.
- ↑ [24] Senthilingam. "Dengue fever: How a mosquito infected millions, and not with malaria". 2016. CNN
- ↑ [25] "Dengue vaccine research". 2016. WHO.
- ↑ [26] Attaran, Off the Podium: Why Public Health Concerns for Global Spread of Zika Virus Means That Rio de Janeiro’s 2016 Olympic Games Must Not Proceed
- ↑ http://www.rio.rj.gov.br/dlstatic/10112/6062171/4159109/denguenotificadosexcetodescartadosMes_2016_21_03_2016.htm
- ↑ http://www.theguardian.com/world/2016/mar/30/brazil-zika-war-virus-military-operation
- ↑ [27] Faria et al. "Zika virus in the Americas: Early epidemiological and genetic findings". 2016. DOI: 10.1126/science.aaf5036
- ↑ http://www.bbc.com/sport/olympics/35444254
- ↑ 32.0 32.1 [28] Zika Virus in Brazil. CDC. 2016
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.

