The Effect of Birthing Method on the Infant Gut Microbiota
Abstract
Birthing methods, both vaginal and cesarean section play an essential role in the development of the infant. There has been recent interest and research surrounding the microbiome of the infant and how the bacteria they possess have a direct link to their delivery. The bacteria the infant ingests from the mother’s birth canal helps to build the immune system and better digest breast milk and solid foods. The changes of the vaginal microbiome during pregnancy give clues as to how similar the microbiome of that infant is and how important it be that the infant receives these bacteria.
The incidence of C-section relates to both the necessity of the procedure in times of emergencies, as well as elective surgery to potentially avoid the possible complications of birth. In the baby who is born via C-section, the microbiome that is developed is much less diverse than the baby born vaginally. These babies are at a higher risk of needing antibiotics, which can decrease bacterial diversity immensely. There is an increased risk for the development of asthma, allergies, obesity and other illnesses and diseases in those babies who are born C-section.
The Human Flora
The human body is made up of trillions of microorganisms (Knight, 2015). that affect both the functional and physiological aspects of the human, as well as their susceptibility to pathogens (Davis, 1996). Development of the human flora begins at childbirth and continues to develop well into adulthood. Bacteria thrive in certain environments, which results in large colonies among different parts of the body. The skin harbors a relatively consistent bacterial flora, with most bacteria remaining on the skin, while some attach to the nasal mucosa or deep hair follicles. In any given human being, there are about 10 trillion human cells to 100 trillion microbial cells and 20,000 human genes versus 20 million microbial genes (Knight, 2015).This equates to an average of three pounds of microbes in a human being with those microbes clearly outnumbering human cells. Having a vast number of microbes is imperative to the health of the human race, as they play a key role in fighting pathogens (Davis, 1996).
The formation of the human microbiota begins during the birthing process, making the vaginal microbiome a key role in that development. The vaginal microbiome is one that remains very subjective, as the bacteria present depends greatly on the woman’s age and race (Akst, 2011). The most commonly found vaginal bacteria is the Lactobacillus.Lactobacilli act as the mediators in the vagina by interacting with the environment to sustain the traditional conditions necessary for the vagina (Nissl, 2014). These bacterium create lactic acid that helps to achieve the natural acidic pH. A disturbed pH prompts the Lactobacilli to produce more lactic acid to preserve the environmental conditions. Maintaining an acidic environment in the vagina helps to protect the woman from potentially pathogenic bacteria. While it has been thought that the Lactobacilli tend to be the most dominant bacteria in the vagina, race may determine how much is actually present. In a study among four hundred women, the majority were found to have a strain of Lactobacillus as the most dominant bacteria in the vagina (Akst, 2014). It was also discovered that Asian women and caucasian women were found to have a higher rate of the bacteria in their vaginal microbiome compared to Hispanic and black women (Akst, 2011). For those women whose vaginal bacteria was not dominated by Lactobacillus, other anaerobic bacteria were found that helped to maintain the acidic environment
(Akst, 2011).
A second microbe that is found in vagina is Streptococcus agalactiae, which is a routinely healthy bacterium. A weakened immune system that results in fewer antibodies to fight the harmful pathogenic characteristics of this bacterium can produce damaging effects on the environment. These bacteria are hemolytic, which classifies them as potentially dangerous to neonates, who are highly susceptible to new microorganisms, who are born from a mother that is colonized (Stevens, 2002), (Kaplan, 2002) .
Another microbe that is commonly found in the vagina is the Gardnerella vaginalis bacteria. While it can sometimes cause harmful effects, such as if it is passed to the newborn through birth, it is also found that in some cases, no harm may be done with a finding that about a quarter of all healthy women harbor this bacteria with no harm (Centers for Disease Control and Prevention, 2014). This bacteria works to elevate the pH of the vagina and change the environment. They are known for creating protective biofilms against antibiotics and activate an inflammatory response that can displace Lactobacilli by raising the pH in the vagina (Saunders et al., 2007)
The Birthing History
The process of giving birth and how the birth happened dates back to hundreds of years ago where little was known about the safety and risks of different birthing methods. In the 1500’s century, cesarean section was a relatively new procedure. It was primitively used as an attempt to save a baby from a mother who was in the process of dying or was already dead, so it was not intended to save the mother’s life when it was first practiced (Sewell, 2013). Many of these C-sections took place on kitchen tables and beds, as access to hospitals was limited and even with access, the idea that the people working in the hospital and the hospital itself spread infection was enough to keep the surgery at home. There was a lot of trial and error as the C-section procedure evolved, including where to make the incision along the uterus and whether or not to leave stitches inside of the body. There were many post-operative infections that put the mother at risk, the most frequent of those being septicemia and peritonitis. As the use of C-section continued to be successful, they became the favored method of delivery instead of undergoing a prolonged or difficult labor (Sewell, 2013). Today, the tendency to have an elected C-section is increasing, as it is believed that it will prevent complications that may occur with the mom or baby (Leggitt, 2013).
In the United States, as of 2013, the number of vaginal births was 2,642, 892, whereas the amount of C-section births was 1,284,339, resulting in 32.7% C-section births (Centers for Disease Control and Preventiona, 2015). Compared to the rate of C-sections in 2010, the rate has decreased slightly from the 32.8% it had been at (Martin, et al., 2012). In 2010, it was the first time since 1996 that the rate of C-sections had decreased. Between the years of 1996 and 2009, the rate of C-section increased by almost 60% (Martin, et al., 2012). According to WHO, a safe rate of C-sections is between 10-15% (Gibbons, et al., 2010). Those countries whose rates are below are said to be underusing C-sections and those above, overusing. In the year 2010, only 14 countries fell within the 10-15% ideal range, whereas 54 countries were considered underutilizing and 69 countries were overusing (Gibbons, et al., 2010). It was concluded that low income and limited access to healthcare were the main reasons for underutilization.
The course of vaginal delivery has also changed with the access to new technology. Having the ability to monitor the fetus while in utero allows for birth to be more flexible with options such as water birth or home birth. In vaginal deliveries today, the use of vacuums and forceps are both still used, although the use has decreased (Leggitt, 2013). Induction of labor is very common today, with about 40% of women being induced. Of that 40%, about 10% of the women have an actual need to be induced (Leggitt, 2013). The overuse of a technique such as induction, shows the impatience that has developed overtime in regards to labor. Although it is sometimes necessary for health reasons, it is similar to the overuse of C-section; waiting to see how the body processes this natural experience may turn into a positive experience.
As the technology and the knowledge base surrounding birth continues to grow, we are constantly learning what may or may not be safe for both the baby and mom during and after childbirth. The history of childbirth and the different methods use contribute significantly to the research that continues today on what becomes important in childbirth. The idea that how a baby is born can greatly impact their life is a relatively new idea that is currently being researched. It may come across that a C-section can prevent potential problems with the baby or even with the mother, but it is becoming clearer that how the baby is born can actually predict what their health may encounter in the future.
Vaginal Birth
Changes of Mother's Microbiota in Pregnancy
https://commons.wikimedia.org/wiki/File:Enterococcus_faecalis_SEM_01_Detail.png
During pregnancy, the body essentially becomes an incubator, hosting a wide variety of bacteria that come and go during the nine months (Koren, 2012). In a study done by Ruth Ley, associate professor at Cornell University, ninety-one different pregnant women were studied, including their diet, any medical complications, and a follow up of the child to four years of age. At the end of them women’s pregnancies, it was discovered there was a decrease in the number of different types of bacteria in the gut and the final trimester microbiota was similar to that of an obese person. To study these effects on the mother, normal nonpregnant mice were given the same bacteria that were found in the third trimester of the mother. Following the administration, the mice gained more weight than the mice that were give the first trimester bacteria despite the fact that both were following the exact same diet. It was observed that the bacteria found in the third trimester are able to extract a larger amount of calories from the food the mother ingests, storing these extra calories as extra weight. Since the calories the mother ingests during pregnancy are also used by the newborn, having the ability to eat less while still consuming enough calories to keep both the baby and mom healthy is very important.
It has also been discovered that during the third trimester of pregnancy, the bacteria may have the effect of increasing inflammation. It was observed that there was an increase in Proteobacteria, which is often found in people who have inflammation in their gastrointestinal system, as well as those who have an imbalance of microbes within their body. In addition, there was a decrease in Faecalibacterium, which is beneficial in decreasing inflammation (Koren, 2012). With an increase of inflammation activating microbes in the gut of the mother, it would be thought that these would be the microbes that would colonize in the newborn first.
The pathway that introduces the mother’s vaginal bacteria into the newborn begins in the baby’s mouth where a big “gulp” of those bacteria enriched vaginal secretions and fecal matter is ingested by the newborn. The most commonly found bacteria in the flora of a vaginally born newborn are Lactobacillus, Bifidobacterium, Escherichia coli, and Enterococcus (Favier, et al., 2002), (Favier, et al., 2003). Bifidobacterium infantis is a microbe that is specific to only the newborn digestive tract. (Matsuki, et al., 2006) They play a key role in fermentation, which produces lactic acid and creates an acidic environment that defends against pathogens (Wall, et al., 2008) E. coli produces K and B vitamins that are then absorbed by the intestines (Park, et al., 2005) Other strains of E. coli can aid in the uptake of nutrients or stimulate the secretion of IgA (Henker, 2008). The Enterococcus species is considered to be a facultative anaerobe whose main role is fermentation of carbohydrates and sugars into lactic acid (Mark, et al., 1998).
In a vaginally born infant, the microbiota is more closely related to the bacteria found in the mother during her first trimester. Although there is a dominance of different bacteria during the third trimester, those microbes found during the first trimester are still present during the time of delivery they are just not as abundant. Due to the very acidic environment of the newborn’s stomach with decreased oxygen, only those microbes who are suited to thrive in these conditions will survive (Parracho, et al., 2007), (Penders et al., 2006). During a 2007 study by Stanford University (Palmer, et al., 2007), infants from birth to one year were observed to analyze the changes in each individual’s microbiota. It was discovered that the gut bacteria found in these babies were very unstructured and unique to one another the only two to have similar microbiota were the only set of twins from the study. It seemed as if the newborn gut sorted through the bacteria it was given in order to assemble the microbiota that would best suit them. It is possible that part of this may be related to the low pH of the infant gut. Although the pH rises as the gut continues past the stomach, it is possible some microbes do not survive in the acidic environment.
GBS in Pregnancy
Group B Streptococcus is an opportunistic pathogen that is found in 25% of all healthy adult women (American Pregnancy Association, 2015). While GBS can affect the woman negatively, the rate of infection among the newborn population is much higher (Centers for Disease Control and Prevention, 2014). GBS can be readily passed to the baby through vaginal delivery making it important that the mother gets screened between 35 and 37 weeks gestation (American Pregnancy Association, 2015). A woman who is found to be GBS positive will receive antibiotics only during the delivery. The antibiotics must not be given too early in labor due to the fact that this bacterium naturally lives in the vagina. Antibiotics given too early allow the bacteria time to grow back before delivery. A baby who is born GBS positive is at high risk for health problems. In the United States, GBS is the leading cause of meningitis and sepsis in newborns (Centers for Disease Control and Prevention, 2014). It can also cause pneumonia, respiratory problems, heart and blood pressure instabilities, and gastrointestinal and kidney problems (American Pregnancy Association, 2015). There is a 1 in 200 chance of the baby contracting GBS if no antibiotics are used during delivery and a 1 in 4,000 chance of contracting it if antibiotics are used (Centers for Disease Control and Prevention, 2014). If a mother is GBS positive, her membranes have not ruptured, and she is not in labor, antibiotics will not be necessary if she is receiving a C-section because the intact membranes give no path for the bacteria to travel to the fetus. The effects of antibiotics will be discussed later in the paper.
Cesarean Section Birth
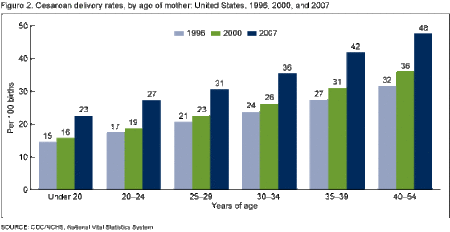
http://www.cdc.gov/nchs/data/databriefs/db35.htm#cesarean
Choosing how to deliver a child is a decision that has many factors. Delivering a child vaginally is generally the goal for childbirth, but other factors contribute. Implications for delivering via cesarean section include: labor that is not progressing, oxygen deprivation to the baby, breech or transverse position of the baby, multiple gestation, placenta problems and previous cesarean section delivery. Although having a C-section for these reasons may be necessary, many women choose to have a C-section to avoid delivery, have a planned delivery, or avoid possible complications of vaginal delivery (Mayo Clinic Staff, 2015).
In a newborn who is delivered via C-section, the microbes that are present vary. In a study where the cord blood of a healthy newborn delivered cesarean, the majority of the bacteria found were from the Enterococcus, Streptococcus, Staphylococcus, and Propionibacterium genera (Jiménez et al., 2005). Both Staphylococcus, and Propionibacterium are generally found in the skin flora. In a baby who is born C-section, their first exposure to bacteria are from the tools that are used for delivery and the skin of the people who touch them. Since they do not pass through the birth canal, they do not get to pick up the bacteria from her vaginal secretions and feces, building a very different microbiota than those babies born vaginally.
Innoculating Newborns with Mother's Vaginal Secretions
With continual research showing the benefits of a vaginal delivery, there has been new research developing in regards to inoculating a neonate born cesarean with the mother’s vaginal secretions. The goal of inoculation would be to provide those babies born via C-section the same bacteria that vaginal birthed babies receive to help build their microbiome. Dr. Maria Gloria Dominguez-Bello who is an associate professor in the Human Microbiome Program at the NYU School of Medicine has begun research on the inoculation process. Initial findings of inoculating newborns with gauze that has been saturated with the mother’s vaginal secretions found that their microbiomes did more closely resemble those who were born vaginally, but it was only a partial match (Goldberg, 2014). The number of bacteria observed from a C-section baby was doubled when they were exposed to their mother’s vaginal secretions, but those who were born vaginally still had six times the number of bacteria. In a vaginal birth, the baby is exposed to the mother’s bacteria for hours. This extended period of exposure gives the bacteria more time to incorporate itself into the neonate, allowing for this process to be maximized. In a cesarean delivery, it is important to note that it is likely that the mother, baby, or both has been exposed to antibiotics to prevent post op infection, possibly killing off any bacteria that had developed.
The NICU
The neonatal intensive care unit is a place for high-risk infants who are extremely vulnerable to infections. Infants who may need admission to the NICU include preterm, complications during delivery that require intervention on the newborn, underdevelopment, congenital anomalies, and any other issue that may compromise their health. Due to the extreme risk of infection because of their very immature immune systems, equipment, techniques, and procedures are done in a clean or sterile matter to essentially eliminate anything that could harm the baby.
In a study researching the neonatal intensive care unit, it was found that the microbial population within the unit closely resembled those found in the gut of premature infants. Many of the premature infants that are placed in the NICU have been delivered by cesarean section and many of the infants have been given multiple antibiotics to prevent infection. The NICU is to remain germ free, so frequent sterilization and disinfecting is performed to keep it this way. In a study performed by the University of California Berkeley (Eisen, 2014), fecal samples from two premature infants were sampled to analyze their microbial population. Samples from the most frequently touched surfaces in the NICU were also taken to compare to the fecal samples. The surfaces swabbed included the sink, feeding and breathing tubes, the hands of healthcare staff and parents, knobs on the incubators, and items from the nurse’s station, such as keyboards, mouses, and cell phones. The two fecal samples were compared to the surface samples and it was found that they were very similar. Among the surface samples, the bacteria collected from the feeding and breathing tubes were the most populated in the infant feces showing that these surface microbes relocated themselves into the newborn gut. Within both samples, it was also found that many of the bacteria harbored resistance genes that raise concern that they could be the same genes that produce antibiotic resistance. If the microbes found in the feces of the NICU patients are similar to those found on the surfaces, it raises a red flag for resistance, as those microbes have already resisted the disinfectants used to maintain sterility. Since all intensive care units are under strict protocol to maintain a sterile environment, it may be a matter of looking into the disinfectants used and changing them to see if that has an impact on reducing those bacteria that are already resistant.
Breastfeeding and Infant Formula
Breastfeeding not only provides countless benefits for bonding between a mother and baby, but it also is provides key components for the neonate. Although breastfeeding is the ideal way to feed the newborn, it is not always possible. Some mothers find it uncomfortable or cannot produce enough milk and some health defects, such as cleft palate, can create difficulties when trying to breastfeeding. Formula is a desirable alternative to breastfeeding, as it provides adequate calories and many are fortified to include components such as iron. With this being said, breast milk is superior choice for feeding the newborn. The components of breast milk include proteins, carbohydrates, antibodies and other immune system components (Sonnenburg, J and Sonnenburg E, 2015). One of the most important components of breast milk is human milk oligosaccharides or HMOs. HMOs are the third most abundant component of breastmilk and have many functions when ingested (Vandenplas, 2002). In the body, HMOs act as receptors against pathogens, preventing attachment to the epithelium in the gut (Jantscher-Krenn, Bode, 2012). The infant cannot necessarily break down the HMOs mechanically, but the microbial population in the infant’s gut can digest these components and collect the energy from this (Sonnenburg, J and Sonnenburg E, 2015). This important component of breast milk has also shown to reach the colon in its original composition, providing benefits that support a healthy gut (Jantscher-Krenn, Bode, 2012). It also serves to prepare the infant for solid foods by feeding the current microbiota and helping to create a population of Bacteroides (De Filippo, 2010). It has also been found that HMOs manage the shift of bacteria that takes place when solid food is introduced. Bacteroides can proficiently consume HMO’s and activate mucus-utilization genes, which absorb HMO’s (Marcobal, 2011). This allows the benefits of HMOs to be utilized by the newborn. When considering the benefits that this community of microbes has on the gut, it has also been discovered that consumption of breast milk has shown to contribute to a decrease the incidence of developing diarrhea, influenza, and respiratory infections during infancy, as well as type 1 diabetes, allergies, and multiple sclerosis (Duke Medicine News and Communication, 2012) .
A newborn’s gut flora is not only determined by their mode of delivery, but also through their feeding habits. The breast milk that a newborn ingests will aid in the development of colonies of essential microbes in the intestinal tract. These microbes facilitate in the absorption of nutrients, as well as immune system development. A study to determine how bacteria grows and develops in milk was performed at Duke comparing infant formula, cow’s milk, and breast milk. Each of the milk samples was incubated with two different strains of E. coli (Duke Medicine News and Communication, 2012). E. coli is a normal bacteria that lives in the human intestines and is beneficial in maintaining a healthy intestinal tract, as well as preventing harm from dangerous strains of E.coli (Centers for Disease Control and Preventionb, 2015). The results from the incubation showed that bacteria in all of the milk samples grew within minutes, but the colonization among each were different. In the breast milk sample, the bacteria created a biofilm (Duke Medicine News and Communication, 2012). Although some biofilms can be harmful, a biofilm within the gut can help to decrease infections and fight off disease (Hook, et al., 2012). In the cow’s milk sample and the infant formula sample, the bacteria grew individually, but there was no protective biofilm formed (Duke Medicine News and Communication, 2012). This study proved that breast milk is not only important for the nutrition and microbes in the gut, but also for those microbes that act as protectors against harmful pathogens.
In a fecal analysis of 98 Swedish infants that tracked them over the first year of life, there was a significant difference in the bacteria’s present in breastfed versus non-breastfed babies (Bäckhed, 2015). In those infants that were not breastfed, it was discovered that their gut bacteria predominantly consisted of Clostridia, further finding <i.Roseburia, Clostridium, and Anaerostipes, which are frequently present in the adult gut microbiota. The breastfed infants were predominantly colonized with Bifidobacterium, which is commonly used in probiotics, and Lactobacillus, which flourishes when breast milk is ingested. By not breastfeeding and in turn developing a microbiota similar to an adult’s, it creates a very different set of microbes in those newborns who are breastfed and those who are not. It raises question as to whether or not breastfeeding allows for a longer evolution of the microbiota and that not breastfeeding shortens the evolution period (Bäckhed, 2015).
The choice to use infant formula occurs for many different reasons some being a necessity while others are preference. With the major difference between breast milk and infant formula being the lack of HMO’s in formula, there have been efforts to create a similar component to add into infant formula. Two components, galacto-oligosaccharides and inulin, have been added to some formulas in hopes to better mimic breast milk (Vandenplas, 2002). Although they do not provide all of the same benefits as breast milk, these added components have shown to promote the growth of Bifidi and Lactobacillus. The bacteria found in the newborn’s intestines are important for preventing infection and promoting healthy growth. Babies that are breastfed inherit this barrier of protection, while formula-fed infants have an ever changing gut microbiota that increases their risk of illness. Breastfed babies naturally have a healthy, beneficial gut microbiota. Those who are formula fed need help with decreasing the amount of illness-associated bacteria and increasing the amount of helpful bacteria (Holscher, 2012).
Probiotics and prebiotics have both been added to infant formulas with the effects being researched. Prebiotics are carbohydrates that maintain their structure through digestion and help to stimulate both growth and activity of the “good” bacteria in the gut (Holscher, 2012). Probiotics are actual live bacteria that also stimulate growth of the beneficial bacteria in the gastrointestinal tract. In a study comparing 172 healthy six-week old infants that were either breastfed, formula fed with prebiotics, or formula fed with probiotics, it was observed that a profound immunity was developed among the probiotic-fed infants, especially among those that were born cesarean. The probiotic formula contained Bifidobacterium animalis subspecies lactis, which is a favorable bacteria. In those infants, there was an increased amount of secretory, anti-rotavirus, and anti-poliovirus specific immunoglobulin A (IgA). Among the babies who were given the prebiotic formula, there was a mild improvement with an increased in some favorable bacteria and a decrease in disease-causing organisms. Incorporating probiotics into formulas have shown to have a positive effect on the newborn, which is very important for those babies who are not breastfed. It will be pertinent to continue researching which probiotics have the most outstanding effects in order to provide the most beneficial bacteria to the newborn.
Antibiotics and the Newborn
Antibiotics are used for a variety of reasons to defend the neonate from potentially harmful pathogenic bacteria. Since C-sections are an invasive procedure, prophylactic antibiotics are commonly used to help prevent infection in the mother, post op. Exposure of antibiotics to the mother also means exposure to the fetus and their bacterial community through the placenta, which puts the newborn at risk for a compromised microbiota. The gut microbes found in all humans play a critical role in cultivating a rapid production of granulocytes, which are white blood cells that focus on fighting infection (Deshmukh, et al., 2014). When a neonate is born with an already antibiotic exposed gut, the body adapts immediately by greatly increasing the amount of granulocytes in preparation of exposure to a vast number and variety of bacterium (Deshmukh, et al., 2014). In addition to exposure in utero, antibiotics are a common medication among babies and children with about one-quarter of all medications given to children falling under the category of antibiotics, one-third of those being unnecessary (Vangay, et al., 2013). This, again, puts the baby or child at risk for depletion of important and beneficial bacteria.
Whether the newborn is exposed to antibiotics directly during the postnatal period or indirectly prenatally, the microbiome changes accordingly. Essentially any kind of medication that enters the mother’s bloodstream will also enter the fetus’s bloodstream via the placenta, increasing the risk of exposure to antibiotics wide open. In a newborn receiving their first round of antibiotics, the diversity in the gut plummets (Sonnenburg, J. and Sonnenburg, E., 2015), putting them at high risk for infection. With a primary goal for antibiotic use to be prevention of further infection, these observations seem to contraindicate the use. When a repeat course of the antibiotics is given, the diversity that initially decreased tremendously does not decrease to the same effect as the first round due to adaptation by the microbiota (Sonnenburg, J. and Sonnenburg, E., 2015). Not only are these antibiotics killing the bacteria that are considered to be harmful, but they are also killing the beneficial bacteria. When given broad spectrum antibiotics, which is a general course of action when a source of infection is unknown, there is no cell specificity when deciding which bacteria should not be killed and which ones should.
Although the bacteria that are destroyed through antibiotics can recover, it is highly unlikely the microbiota will ever be the same (Sonnenburg, J. and Sonnenburg, E., 2015). In addition, the use of antibiotics in a newborn has been linked to increased risk for asthma, eczema, and obesity (Trasande, 2013) (Hoskin-Parr, 2013) . In a comparison done between children who received antibiotics as an infant and those who had no exposure to antibiotics there was a significant difference in their weights (Trasande, 2013). The children who had received antibiotics at six months or younger weighed much more than those who did not have any. Those who had received antibiotics after six months of age still weighed more than those who did not, although it was not as significant. In adults, fecal transplants have been utilized in treating severe bacterial infections (Deshmukh, et al., 2014). with a 92-95% success rate among a 200 case reports (The Fecal Transplant, 2015). Fecal transplants have not been performed in newborns, as it is difficult to determine whether a newborn that is critically ill is truly infected with bacteria (Deshmukh, et al., 2014). Until there is a more protective and successful method to treat a newborn without harming the microbiota, antibiotics will be administered whenever necessary (Deshmukh, et al., 2014).
How Does Delivery Method Affect the Child's Life
Cesarean section delivered babies has shown that this delivery method impacts their lives far beyond infancy. Research has proven that allergies, asthma, obesity, and eczema are a few of the complications that can result from a cesarean birth. In the first two years of life, the guts of C-section babies are much less diverse, with a specific low diversity among Bacteroidetes (Jakobsson, et al., 2013) This particular bacteria aid in protection against allergies so a decreased diversity results in the inability to fight allergies. Babies who are born via C-section are five times as likely to develop allergies than those born vaginally when they are exposed to common allergens found in houses (Johnson, 2013). Vaginally born babies have shown to have an increase in the blood plasma levels of Th1 cells (Jakobsson, et al., 2013). These Th1 cells can help to obstruct allergic immune responses, which decrease their susceptibility to allergies. In addition, the cesarean born babies have an “at risk” pattern of microbes in their GI tract that makes them more susceptible to progressing to the development of antibody IgE (Johnson, 2013). Immunoglobulin E plays a major role in allergic diseases, including asthma. The early exposure that a baby gets when born vaginally affects the development of the immune system greatly (Johnson, 2013). Those who are born vaginally have a much more diverse group of bacteria in the gut, which gives them more protection against pathogens and allergens. (Jakobsson, et al., 2013).
In addition to allergies, it has been found that C-section is linked to obesity. These babies are at a 46% higher risk of developing childhood obesity than those who were born vaginally. (Mueller, et al., 2014). As discussed previously, many babies born via C-section are exposed to antibiotics at some point, whether it is in utero or after birth, which increases their risk of obesity. It has also been observed that in general, mothers who received C-sections were at a higher weight and their babies were also at a higher weight compared to those born vaginally (Huh, et al., 2012). C-section was also linked to a doubling in the odds of a child becoming obese by the age of three, which also included an increase in BMI and an increase in skinfold thickness (Huh, et al., 2012). In a study involving mice, it was observed that in addition to gaining weight readily, the mibcrobiota of these mice paralleled those of an obese person (Cho, 2012). Not only was the weight of these mice affected, but they also had an increase in bone density and specific hormones involved with metabolism were altered. This observation creates a possible connection between cattle that are given antibiotics to gain weight and children who receive antibiotics and later develop obesity (Sonnenburg J., and Sonnenburg E., 2015).
When comparing and contrasting the information and data that has been collected, it seems that there is a clear distinction between how the baby is born and how it affects their life. With this being said, it is hard to tell if these effects are skewed based off of the fact that most C-sections incorporate antibiotics, whether it is to mom, baby, or both. It would seem that the antibiotics have a lasting effect, but from the research it seems that whether or not the baby gets that gulp of bacteria during birth has a greater effect.
Conclusion
The research surrounding cesarean born infants versus vaginally born infants has brought significant information to the surface. The method of a vaginal birth allows for the infant to pass through a birth canal that is abundant in bacteria that prove to be significant in the infant’s life. In a baby born C-section, the baby does not get to pass through the birth canal and is not exposed to the mother’s bacteria and instead often collects the bacteria of the mom’s skin, operating room surfaces and surgeons. C-sections are indicated for emergencies in which the mother or baby may not survive a vaginal birth, though many women are electively having a cesarean in hopes that it will prevent complications.
During breastfeeding, the bacteria that are found in the infant’s gut play a key role in breaking down the components of the milk, especially HMO’s, which are not readily broken down. Many infant formulas have begun to add similar components to HMO into their formulas, but the effects have not yet shown the same benefits as breast milk. The additive probiotics and prebiotics have shown a significant increase in the immunity among the infant, but it still does not compare to the immunity developed through breast milk.
The use of antibiotics during pregnancy or in the infant following birth has shown to have many long-lasting effects. There are many indications for antibiotic use in the pregnant mother, but the transfer of these to the fetus through the placenta can damage their gut bacteria development, even after birth. In those infants who are given antibiotics following birth, it creates a risk for depletion of the bacterial diversity within the gut. Although there are serious indications for antibiotics in the newborn, there is an overuse of them in newborns that may not need them indefinitely. Antibiotics in addition to C-section deliver both have a lasting effect on the child, putting them at risk for chronic illnesses and conditions. Due to the decreased diversity in the gut of these children, they have an increased risk for developing allergies, asthma, obesity, eczema and other health related difficulties.
As the research and data surrounding this topic continues to grow, it has already shown significant outcomes in regards to how one is birthed. Though the importance of bacteria and the microbiome in a human being is often times unknown or misunderstood, this remains something that needs to be talked about more among health care and families. Because this is a new idea to most expecting mothers and there are not many options as of now, I think there needs to be more research done on how these important vaginal secretions could be incorporated into those who are born cesarean. Inoculation of the newborn has been the most promising method, but there has not been much research or success in regards to this method. Knowing that the delivery method inoculates the infant with microorganisms that will have an important impact on their life becomes an important piece of knowledge.
References
Akst, J. (2014, May 19). Characterizing the “Healthy” Vagina.
Centers for Disease Control and Preventiona. (2015, July 17). Births - Method of Delivery.
Centers for Disease Control and Preventionb. (2015, November 6). E. coli, General Information.
Centers for Disease Control and Prevention. (2014, June 1). Group B Strep (GBS).
Cho, I., et al. “Antibiotics in Early Life Alter the Murine Colonic Microbiome and Adiposity.” Nature 488.7413 (2012): 621-26. Print.
De Filippo, C., et al. “Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa.” Proc Natl Acad Sci U S A 107.33 (2010): 14691-96. Print.
Deshmukh, H., et al. (2014). The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nature Medicine Nat Med, 20, 524-530. doi:10.1038/nm.3542
Favier CF, De Vos WM, Akkermans AD. (2003) Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe, 9:219-229.
Favier CF, Vaughan EE, De Vos WM, Akkermans AD. (2002) Molecular monitoring of succession of bacterial communities in human neonates. Applied and Environmental Microbiology, 68:219-226.
Gibbons, L., Belizán, J., Lauer, J., Betrán, A., Merialdi, M., & Althabe, F. (2010). The Global Numbers and Costs of Additionally Needed and Unnecessary Caesarean Sections Performed per Year: Overuse as a Barrier to Universal Coverage. Retrieved from: http://www.who.int/healthsystems/topics/financing/healthreport/30C-sectioncosts.pdf/
Henker J. (2008) Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. The Pediatric Infectious Disease Journal, 27:494-498.
Hook, A., Chang, C., Yang, J., Luckett, J., Cockayne, A., Atkinson, S., Mei, Y., Bayston, R., Irvine, D., Langer, R., Anderson, D., Williams, P., Davies, M., Alexander, M. “Combinatorial Discover of Polymers Resistant to Bacterial Attachment.” Nature Biotechnology, 2012. DOI:10.1038/nbt.2316
Hoskin-Parr, L., et al. “Antibiotic Exposure in the First Two Years of Life and Development of Asthma and Other Allergic Diseases by 7.5 Yr: A Dose- Dependent Relationship.” Pediatr Allergy Immunol 24.8 (2013): 762-71. Print.
Huh, S., Rifas-Shiman, S., Zera, C., Edwards, J., Oken, E., Weiss, S., & Gillman, M. (2012). Delivery by caesarean section and risk of obesity in preschool age children: A prospective cohort study. Archives of Disease in Childhood, 97(7), 610-616. doi:10.1136/archdischild-2011-301141
Jakobsson, H., et al. (2013). Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut, 63(4), 559-566. doi:10.1136/gutjnl-2012-303249
Jantscher-Krenn, E., & Bode, L. (2012, February 1). Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Retrieved December 10, 2015, from http://www.ncbi.nlm.nih.gov/pubmed/22350049
Jiménez, E., et al. (2005). Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Current Microbiology, 51(4), 270-274. Retrieved from: http://link.springer.com/article/10.1007/s00284-005-0020-3
Koren, O., et al. “Host Remodeling of the Gut Microbiome and Metabolic Changes During Pregnancy.” Cell 150.3 (2012): 470-80. Print.
Matsuki T, Watanake K, Tanaka R. (2003) Genus- and Species- specific PCR primers for the detection and indentification of Bifidobacteria. Current Issues Intestinal Microbiology, 4:61-69.
Mayo Clinic Staff. (2015). C-section. Mayo Foundation for Medical Education and Research.
Mueller, N., et al. (2014). Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes Relat Metab Disord International Journal of Obesity, 665-670. doi:10.1038/ijo.2014.180
Nissl, J. (2014, September 25). Vaginal Wet Mount. Healthwise, Incorporated.
Palmer, C., et al. “Development of the Human Infant Intestinal Microbiota.” PLoS Biol 5.7 (2007): e177. Print.
Parracho H, McCartney A, Gibson G (2007) Probiotics and prebiotics in infant nutrition. Proceedings of the Nutrition Society, 66:405–411
Park H, Shim SS, Kim SY, Park JH, Park SE, Kim HJ, Kang BC, Kim CM. (2005) Molecular analysis of colonized bacteria in a human newborn infant Gut. The Journal of Microbiology, 43:345-353.
Penders J, Thijs C, Vink C, Stelma F F, Snijders B, Kummeling I, van den Brandt P A, Stobberingh E E. (2006) Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics, 118:511-521.
Sonnenburg, J., & Sonnenburg, E. (2015). Assembling Our Lifelong Community of Companions. In The good gut: Taking control of your weight, your mood, and your long-term health (p. 45-57). New York, New York: Penguin Publishing Group. Print.
Stevens, D., Kappen, E. (2000). Streptococcal infections: Clinical aspects, microbiology, and molecular pathogenesis. New York: Oxford University Press.
The Fecal Transplant Foundation. (2015). Frequently Asked Questions.
Trasande, L., et al. “Infant Antibiotic Exposures and Early-Life Body Mass.” Int J Obes (Lond) 37.1 (2013): 16-23. Print.
Vandenplas, Y. (2002). Oligosaccharides in infant formula. British Journal of Nutrition, 87, pp S293-S296. doi:10.1079/BJN/2002551.
Vangay, P., Ward, T., Gerber, J., & Knights, D. (2013). Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host & Microbe, 17(5), 553-564. doi:10.1016/j.chom.2015.04.006
Wall R, Hussey SG, Ryan CA, O’Neill M, Fitzgerald G, Stanton C, Ross RP. (2008) Presence of two Lactobacillus and Bifidobacterium probiotic strains in the neonatal ileum. The ISME Journal, 2:83-91.
Edited by Meryl Jones, student of Rachel Larsen at the University of Southern Maine