The Responses of Cyanobacteria to UV-B Irradiation
Introduction
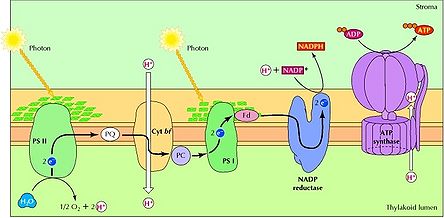
Cyanobacteria are a deep branching phylum of prokaryotes that are of incredible importance to the biosphere. These ancient organisms perform photosynthesis through the use of photosystems (PS) I and II, a design unique among photosynthesizing bacteria and archaea (Figure 1). Pigments such as phycocyanin and chlorophylls a and b are embedded in the cyanobacterial thylakoid membrane. In particular, phycocyanin is associated with cyanobacteria, as it is responsible for the blue-green appearance for which these organisms are named. Pigments absorb wavelengths of light from the visible spectrum of 400 to 700 nm [1]. When energy from light is captured, H2O or H2S is split. When water is the substrate, this reaction releases oxygen gas, protons (H+) and donates an excited electron to PS II. As this initial electron relaxes back to its ground state, it passes its energy to electrons in adjacent atoms until the reaction center of PS II is reached. From this point, electrons reduce various proteins in PS II, allowing H+ to be pumped across the thylakoid membrane to produce a chemical gradient. The potential energy stored in this gradient is then harnessed to make a usable energy source for the cell in the form of ATP. Even more energy is stored when a second electron from PS I is excited. The protein ferredoxin can then produce the energy carrier NADPH.
It is important to note the production of oxygen gas (O2) that occurs during photosynthesis in cyanobacteria. As it turns out, cyanobacteria are the only known bacteria that produce O2 . The evolution of this type of metabolism probably caused the increase of oxygen in Earth’s early atmosphere. Thus, without the presence of cyanobacteria or a similarly photosynthesizing predecessor, aerobic life as we know it could not have evolved.
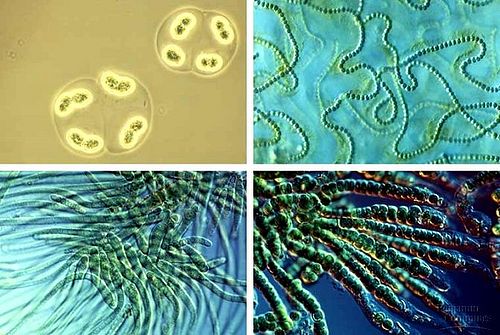
Today, cyanobacteria continue to act as important contributors to the air we breathe and provide a number of other important services to ecosystems. The diverse forms seen amongst species of cyanobacteria allow this phylum to fill key roles in a range of ecosystems including the most extreme aquatic and terrestrial environments on Earth (Figure 2). Cyanobacteria have been found to act as a primary source of usable nitrogen to living organisms. Most species of cyanobacteria are capable of fixing nitrogen, converting atmospheric nitrogen into nitrate or ammonia. In habitats like those found in arctic regions where nitrogen is the most common limiting element, the presence of cyanobacteria accounts for up to 80% of the total annual nitrogen input [18]. The growth of organisms in these regions would be severely retarded without the nitrogen contributions of cyanobacteria. Similarly, cyanobacteria compose a large portion of microbial biomass and productivity in many ecosystems on which a multitude of higher organisms depend [18].
Given these essential functions of cyanobacteria in ecosystems, it is important to understand how these organisms are adapted to their environment and how global change might impact them. The level of ozone-depleting gases in our atmosphere has increased dramatically in Earth’s recent history due to anthropogenic emissions. With a reduction of the ozone layer, organisms on Earth are being exposed to an increased level of ultraviolet (UV) radiation. UV light has shorter wavelengths (290-400 nm) than visible light, meaning that it has higher energy [1]. Exposure to this high-energy irradiation can lead to the damage of proteins and DNA, as well as changes in growth, cellular differentiation, photo orientation, and the motility of cells [7]. UV-A (320-400 nm) radiation is not impacted by the presence of the ozone layer, and thus exposure to this form of UV light will not change due to ozone depletion [7]. However, UV-B (290-320 nm) photons are typically absorbed by the protective ozone layer, so a decrease in ozone will lead to an increased amount of UV-B hitting Earth’s surface [7]. This will be of particular importance to cyanobacteria, as these organisms depend on near-constant exposure to sunlight for survival. Accordingly, cyanobacteria have been found to poses mechanisms to avoid UV-B rays, defend against them, and repair any damage that they might cause to cellular components [7].
Cellular Damage Caused by UV-B Irradiation
UV-B irradiation has been found to have a large number of damaging effects on cyanobacteria and many other organisms. While a small amount of potentially toxic forms of oxygen are normally produced within cyanobacteria, uncommonly high levels of UV light can lead to the creation of an increased number of reactive oxygen species (ROS) from excited photosynthetic pigments [12]. These ROS can cause oxidative damage to polyunsaturated fatty acids in the cellular membrane and breaks in DNA strands as seen in Anabaena sp. [12]. Proteins and DNA absorb UV-B irradiation, which makes them common targets for damage [7]. It has been found that UV-B wavelengths often harm the D1 reaction center protein of PS II, Rubisco, phycobili proteins (like phycocyanin) and nitrogenase [7]. When DNA absorbs UV-B photons, double-stranded breaks, single-stranded breaks, DNA-protein cross links, cyclobutane dimers, and pyrimidine-(6,4)-pyrimidone photoproducts have all been shown to occur [7]. The energy that must be used to protect against and repair this damage impacts the cells’ energy budget. Organisms affected by UV-B exposure have thus been shown to grow more slowly and replicate less frequently than those animals with less of a UV-B burden [11,6].
Motility in Response to UV-B Irradiation
Gliding
Cyanobacteria can often evade the damaging effects of UV-B irradiation by moving. Some species form mat communities in soils by migrating down to the level at which each species can absorb the maximum amount of light and receive the minimum amount of threat from UV irradiation [14]. Because even closely related species of cyanobacteria show a large degree of variability in their sensitivity to UV light [14], mat communities tend to show distinct layers of cyanobacteria. Layers closer to the surface will consist of those species that have a higher tolerance for UV-B irradiation than the species found in lower layers. In fact, it has been found that this distinct layering is lost when cyanobacteria are grown in the absence of UV-B radiation [15]. Likewise, aquatic cyanobacteria can sink to lower levels in the water column to avoid intense radiation [7]. Moving up and down occurs daily in some species like Oscillatoria sp. and Spirulina cf. subsala to avoid the higher light intensity of the afternoon [7,9], whereas other species may only respond to long-term changes. Migration, however, comes at a price. Moving to areas with less light penetration can lead to a decrease in productivity of these photosynthetic organisms [2].
Gas Vesicles
The motility of cyanobacteria is based on the little-understood method of gliding and the simple concept of floating. Gliding involves no obvious change in cell shape or external organelle, and may be related to internal rotation or contraction of some unidentified cell components [5]. This movement can occur over semi-solid or solid surfaces with a film of water on top [5]. It has been shown that cyanobacteria like the species Microcoleus chthonoplastes will move to less intensely irradiated areas when exposed to UV-B light[2,14], indicating that these organisms are photoactive [5]. Despite this similarity in response by different species of cyanobacteria, it has also been shown that the rate at which species react and glide away is variable [14]. When in the water column, gliding becomes less useful, and cyanobacteria rely on gas-filled vesicles to maintain buoyancy. Cyanobacteria are capable of changing their buoyancy by altering the volume of their gas vesicles as a response to environmental conditions including light intensity [4]. Altering gliding patterns and buoyancy based on the strength of UV-B radiation is thus an important way in which cyanobacteria can avoid UV damage.
UV-B Absorbing Compounds
Mycosporine Amino Acids
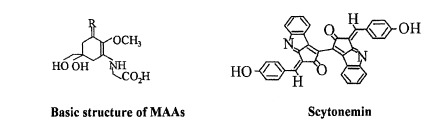
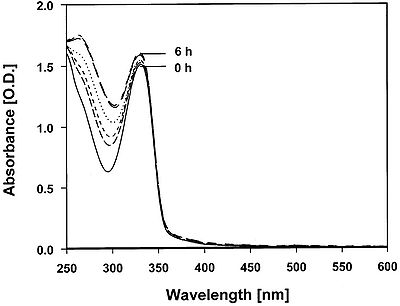
Cyanobacteria can protect themselves against UV-B radiation that does reach the cell by removing the dangerous rays before they can cause damage. UV-absorbing molecules are one way in which cyanobacteria can defend against UV-B photons (Figure 3). Mycosporine Amino Acids (MAA) are water soluble cyclohexanones that are linked to amino acids or amino alcohols [7]. Because MAA absorb wavelengths between 310 nm and 360 nm, these compounds can absorb UV-B before it can damage other important cellular structures [7]. It has been found that MAA in Gracilaria cornea have the potential to remain stable under high temperature conditions for up to 6 hours, making them excellent compounds for dealing with UV-B stress (Figure 4) [17]. MAA block three out of ten photons from being absorbed by proteins or DNA [11]. The higher the concentration of MAA in a cell, the more resistant to UV-B irradiation a species of cyanobacteria tends to be [11]. Heightened concentrations of MAA have been found to arise in response to UV-B irradiation, but not UV-A exposure [6]. However, there seems to be a limit on the amount of MAA a cell can contain [15], therefore placement of the MAA is important as well. MAA located in the cytoplasm have been found to absorb 10-26% of UV-B photons, whereas MAA located in the extracellular glycan absorbs closer to 67% of UV-B photons [7]. This is presumably due to the fact that photons are absorbed before UV-B irradiation gets a chance to enter the cell. While MAA plays a significant role in defense against UV-B irradiation, it is thought that sunscreen may not be the only role of MAA. It is suspected that these molecules might serve as antioxidants to control ROS produced from UV exposure, transfer energy from UV light to PS reaction centers for photosynthesis, or assist with osmotic regulation [16].
Scytonemin
Scytonemin is a pigment that absorbs a maximum wavelength of 370 nm [7],enabling this compound to serve a similar sunscreen function as MAA. This dimeric pigment is a yellow-brown color and is lipid soluble [7]. Researchers have observed an increase in the production of scytonemin with exposure to UV-A light and weakly with UV-B irradiation [6]. Scytonemin is commonly found in the cyanobacterial sheath where it captures approximately 90% of UV-A photons before they can enter the cell [16]. This pigment can make up as much as 5% of cellular dry weight, and degradation within the cell is not rapid [16]. After synthesis of this pigment, the cell need not invest any more energy in order for scytonemin to screen UV photons effectively [16]. These characteristics of scytonemin suggest that the compound plays a primary role as a UV-A sunscreen [6]. The role of scytonemin as a sunscreen has been observed in the terrestrial cyanobacterium Chlorogloeopsis sp [10]. Because of the abundance of scytonemin in cells and prevalence of the pigment across species, it is thought that scytonemin may have evolved early in the history of cyanobacteria. This adaptation would have allowed this phylum to move into shallow waters and terrestrial habitats where exposure to UV light was more intense [16]. A combination of scytonemin and MAA in cells appears to optimize protection against UV-B photons [7].
Extracellular Polysaccharides
In order to enhance the effectiveness of MAA and scytonemin, cyanobacteria produce bacterial extracellular polysaccharides (EPS). These EPS are located in the sheath of a cyanobacterium, and help to form a boundary between the environment and the cellular interior [6]. UV-B absorbing compounds, including some oligosaccharides, can then attach among this matrix of EPS and prevent harmful photons from reaching the inside of the cell [6]. It is again observed that in species such as Nostoc commune an increase in UV-B irradiation can stimulate the production of EPS in order to defend the cyanobacterium from UV-B damage (Figure 5) [6].
Defense Against Reactive Oxygen Species
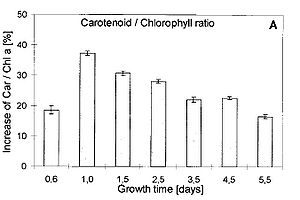
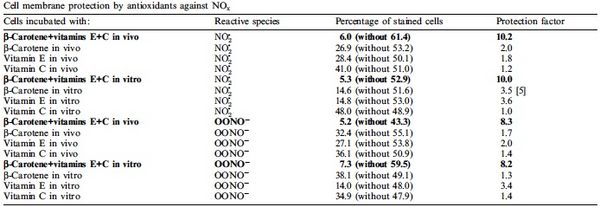
When UV light penetrates a cell and ROS production increases, cyanobacteria are equipped to remove these dangerous molecules with the use of antioxidants. Some antioxidants are scavenging enzymes that include superoxide dismutase, peroxidases, reductases and catalase [12]. These enzymes bind ROS, yielding them harmless or help slow or prevent oxidative reactions from occurring. There are also non-enzymatic antioxidants within the cell that include ascorbate (vitamin C) and the pigments known as carotenoids [12]. Ascorbate is typically available at high concentrations within a cell to reduce ROS to less harmful products [12]. Carotenoids increase in concentration rapidly as UV-B irradiation increases in Nostoc commune (Figure 6) [3]. Once abundant, carotenoids can remove the singlet oxygen and triplet chlorophyll caused by the intense UV light, and can reduce lipid peroxidation by quenching [7]. It has also been found that antioxidant activity is improved with carotenoids and vitamins E and C working together (Figure 7). As carotenoids quench ROS, charged carotene radicals are formed in the pigment [3]. These radicals are repaired when vitamins are attracted by their charge and transfer an electron to the damaged carotenoid [3]. Thus, an abundance of both carotenoids and vitamin E and C lead to a more effective removal of ROS from cyanobacteria.
Cellular Maintenance
Rapid Degradation and Replacement
Despite the numerous ways in which cyanobacteria can avoid and defend against UV-B irradiation, the constant exposure of these organisms to sunlight means that damage does sometimes occur. Cyanobacteria deal with this damage in ways similar to other cells. Damaged cells must either salvage the broken cellular component or replace it entirely. Often times cyanobacteria are found to produce and replace the proteins that are most effected by UV-B photons. As mentioned above, one such protein is the D1 reaction center protein found in PS II. D1 is frequently irreparably damaged by ROS. Without the ability to replace damaged D1 quickly, cyanobacterial photosynthetic rates are reduced [13]. While there is some evidence for an ftsH protease interacting with D1 to degrade this protein [13], the details of the breakdown process are still unclear [13]. D1 has a protruding N-terminus that might be involved in the recognition of damaged D1 by ftsH (Figure 8). Komenda et al. (2007) tested this hypothesis by creating mutant cyanobacterial strains of the species Synechocystis sp. with varying N-terminus residues [13]. They found that some of these mutants inhibited the synthesis of D1 in some cases and prevented D1 removal by ftsH in others [13]. These results indicate that the D1 N-terminus does play a role in D1 degradation, although much is left to be resolved concerning this process [13]. At the conclusion of D1 degradation, de novo synthesis of D1 must occur and fill the place of the destroyed protein. All of these steps come at a large cost to the cell; however, functioning D1 is crucial for cyanobacterial survival.
Repair Mechanisms
To avoid the expense of replacing all damaged cell components, DNA and proteins can be fixed by means of photoreactivation, excision repair, and postreplication repair (SOS repair) [7]. These mechanisms have been well studied in the model organism Escherichia coli, but are found in cyanobacteria as well [7].
Photoreactivation
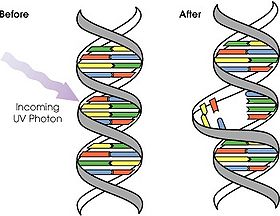
Photoreactivation occurs when UV-B absorbance by DNA causes cyclobutane-type pyrimidine dimers (Figure 9)[7]. The presence of UV light and blue light activates a DNA repair enzyme known as photolyase [7]. Using light energy, photolyase binds the dimer bulge in DNA and can then convert this dimer back into monomers by breaking the bonds of cyclobutane [8].
Excision Repair
Unlike photoreactivation, excision repair is a light independent DNA repair mechanism [7]. In this type of maintenance, damaged DNA can be recognized similarly from the bulges that occur when adjacent bases bond and form cylobutane-type dimers. The enzyme protease can then cut out the dimerization. The resulting gap is filled in anew when DNA polymerase I uses the complementary strand as a template for DNA synthesis. Repair is completed when the enzyme ligase patches the bases from the old strand to the bases in the repaired section together. This is the much more general form of DNA repair, being found in cells of many different phyla.
Postreplication Repair
Postreplication repair is used to produce unharmed complimentary DNA strands from damaged template DNA when DNA polymerase is blocked from functioning by dimers. Activation of the RecA protein by a build up of single-stranded DNA regions is the first step in SOS repair [7]. RecA cleaves the LexA repressor from the SOS operator, allowing SOS gene expression [7]. SOS proteins then conduct an error prone system of repair in the attempts to salvage DNA by making undamaged complimentary strands. Replicating proteins will skip over dimers and continue replicating both strands of DNA at the replication fork. The gap in the complimentary daughter strand can then be filled by splicing in the proper bases from the mother compliment strand (if undamaged) of the mutated template DNA. The gap left in the mother compliment strand can then be repaired with methods similar to those seen in excision repair. In this way, the cell can make a desperate attempt to salvage its DNA sequence after extreme damage has occurred.
Conclusion
Cyanobacteria have a wide range of strategies to avoid, protect against, and fix damage caused by UV-B irradiation for a life in the sun. These mechanisms likely evolved early in Earth’s history and have helped cyanobacteria to become one of the most dominant microbial phylums seen in ecosystems today. More importantly, adaptations to UV-B irradiation may have played a major role in allowing life to move in to more sun-drenched terrestrial ecosystems.
Despite the fact that mechanisms to survive UV-B irradiation have brought success to cyanobacteria in the past, there are new fears that the rapid atmospheric changes brought about by human activity will overwhelm these phototrophs’ defenses. Although humans have reduced the production of atmosphere-degrading molecules such as halocarbons in recent efforts to be more eco-friendly, the detrimental gases we have already released are long lived [18]. This means that the damage we have done will continue to have effects on Earth’s ozone layer long after we have cleaned up our act. With higher levels of UV-B light hitting Earth’s surface, we must consider new concerns relating to the well being of cyanobacteria as important contributors to the biosphere. The responses that species currently exhibit to this environmental stress may not be adequate in times to come. The farther cyanobacteria migrate and the more MAA, scytonemin, EPS, scavenging proteins, and carotenoids that are produced, the less these organisms will be able to grow and divide. Similarly, an increase in the energy allocated towards the replacement and/or repair of damaged proteins and DNA would lead to a decrease in productivity of these organisms. These efforts to escape or control damaging UV photons could reduce the population levels of some species of cyanobacteria. Alternatively, increased cell death from overexposure to UV would have similar effects on cyanobacerial abundance in ecosystems. The latter scenario is particularly unnerving, as this would indicate that climatic conditions are so altered that repair mechanisms have ceased to function effectively [18].
Changes in cyanobacterial populations could have large effects on many ecosystems. As cyanobacteria are primary producers, many other organisms rely on them as a source of energy and nutrients. The biomass and energy available at this level of life dictates the energy and biomass that can be extended to higher life forms. Further, the aforementioned importance of cyanobacteria as nitrogen fixers in terrestrial environments indicates that a reduction of this phylum would severely inhibit the growth and perhaps the survival of a large number of species from a variety of taxons. This would be especially devastating to the many plant species with which species of cyanobacteria have symbiotic relationships. With these things in mind, it is important that we continue to monitor levels of UV-B irradiation and the responses of cyanobacterial species. This is particularly true for the most UV-sensitive cyanobacteria, as they will be under the greatest degree of stress. Changes in these communities may help us predict how ecosystems might be altered and help us to design plans to conserve them. Thus, further studies on the effects of UV-B light on cyanobacteria will be of great value.
References
1. Allen, J. “Ultraviolet Radiation: How it Affects Life on Earth”. Earth Observatory. 2001. http://earthobservatory.nasa.gov/Features/UVB/.
2. Bebout, B.M., Garcia-Pichel F. “UV B-induced vertical migration of cyanobacteria in a microbial mat”. Applied Environmental Microbiology. 1995. Volume 61 (12). p. 4215-4222.
3. Bohm, F., Edge, R., McGarvey, D.J., Truscott, T.G. “β-Carotene with vitamins E and C offers synergistic cell protection against NOx”. Federation of the Societies of Biochemistry and Molecular Biology. 1998. Volume 436. p. 387-389.
4. Brookes, J.D., Ganf, G.G. “Variations in the buoyancy response of Microcystis aeruginosa to nitrogen phosphorous and light”. Journal of Plankton Research. 2001. Volume 23 ()12). p. 1399-1411.
5. Burchard, R.P. “Gliding Motility of Bacteria”. BioScience. 1980. Volume 30 (3). p. 157-167.
6. Ehling-Shulz, M., Bilger, W., Scherer, S. “UV-B-Induced Synthesis of Photoprotective Pigments and Extracellular Plysaccharides in the Terrestrial Cyanobacterium Nostoc commune”. Journal of Bacteriology. 1997. Volume 179 (6). p. 1940-1945.
7. Ehling-Shulz, M., Scherer, S. “UV Protection in cyanobacteria”. European Journal of Phycology. 1999. Volume 34. p. 329-338.
8. Frohnmeyer, H., Staiger, D. “Ultraviolet-B Radiation-Mediation Responses in Plants. Balancing Damage and Protection”. Plant Physiology. 2003. Volume 133 (4). p. 1420-1428.
9. Garcia-Pichel, F., Mechling, M., Castenholz, R.W. migrations of microorganisms within a benthic, hypersaline mat community.”. Applied and Environmental Microbiology. 1994. Volume 60. p. 1500-1511.
10. Garcia-Pichel, F., Sherry, N.D., Castenholz, R.W. [“Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial bacteria Chlorogloeopsis sp.”]. Photochemistry and Photobiology. 1992. Volume 56. p. 17-23.
11. Garcia-Pichel, F., Wingard, C.E., Castenholz, R.W. “Evidence Regarding the UV Sunscreen Role of a Mycrosporine-Like Compound in the Cyanobacterium Gloeocapsai sp.”. Applied and Environmental Microbiology. 1993. Volume 59 (1). p. 170-176.
12. He, Y., Häder, D.P. “Reactive oxygen species and UV-B: effect on cyanobacteria”. Photochemical and Photobiological Sciences. 2002. Volume 1. p. 729-736.
13. Komenda, J., Tichy, M., Prasil, O., Knoppova, J., Kuvikova, S., de Vries, R., Nixon, P.J. “The Exposed N-Terminal Tail of the D1 Subunit Is Required for Rapid D1 Degredation during Photosystem II Repair in Synechocystis sp PCC 6803”. The Plant Cell. 2007. Volume 19. p. 2839-2854.
14. Quesada, A., Vincent, W.F. “Strategies of adaptation by Antarctic cyanobacteria to ultraviolet radiation”. European Journal of Phycology. 1997. Volume 32. p. 335-342.
15. Sheridan, R.P. “Role of Ultraviolet Radiation in Maintaining the Three-Dimensional Structure of a Cyanobacterial Mat Community and Facilitating Nitrogen Fixation”. Journal of Phycology. 2001. Volume 37. p. 731-737.
16. Sinha, R.P., Klisch, M., Gröniger, A., Häder, D.P. “Responses of Aquatic Algae and Cyanobacteria to Solar UV-B”. Plant Ecology. 2001. Volume 154. p. 221-236.
17. Sinha, R.P., Klisch, M., Gröniger, A., Häder, D.P. "Mycosporine-like amino acids in the marine red alga Gracilaria cornea — effects of UV and heat". Environmental and Experimental Botany. 2000. Volume 43. p.33-43.
18. Solheim, B., Zielke, M., Bjerke, J.W., Rozema, J. “Effects of Enhanced UV-B Radiation on Nitrogen Fixation in Arctic Ecosystems”. Plant Ecology. 2006. Volume 182. p. 109-118.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2010, Kenyon College.