The Role of Bacteria in the Health Potential of Yogurt
Introduction
Yogurt is a widely enjoyed dairy product that is essentially an altered form of milk containing waste products from fermentation. The lactic acid that is produced from the fermentation of lactose contributes to the sour taste of yogurt by decreasing pH and allows for the characteristic texture by acting on the milk proteins (Zourari, Accolas, & Desmazeaud, 1992). Yogurt has been continually studied for its health benefits, particularly from the addition of probiotics. Current research has been investigating how to improve yogurt both in terms of its potential as a healthy food and as an appetizing product that appeals to the general population.
The Biochemistry Behind Yogurt
Yogurt is a product of the acidic fermentation of milk. The lactose in the milk is converted to lactic acid, which lowers the pH. When pH drops below pH 5, micelles of caseins, a hydrophobic protein, loses its tertiary structure due to the protonation of its amino acid residues. The denatured protein reassembles by interacting with other hydrophobic molecules, and this intermolecular interaction of caseins creates a structure that allows for the semisolid texture of yogurt (Zourari, Accolas, & Desmazeaud, 1992).
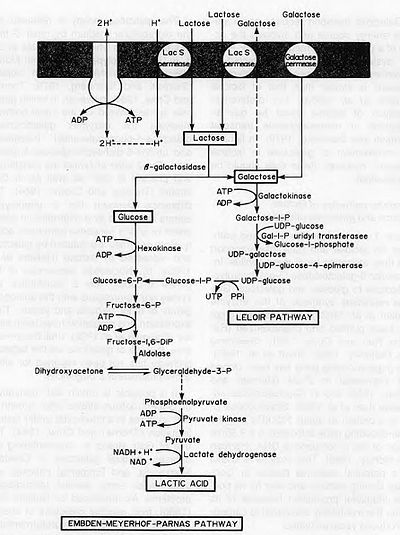
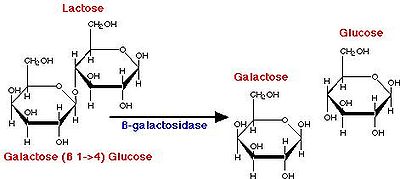
Yogurt production begins with the breakdown of lactose into glucose and galactose (Fig. 2), a process catalyzed by β-galactosidase. The glucose produced from this catabolic step then enters glycolysis, producing pyruvate. It has been proposed that yogurt bacteria utilize the Embden-Meyerhof-Parnas pathway of glycolysis (Fig. 3). Pyruvate then enters lactate fermentation, also known as homolactic fermentation, as it produces only lactic acid molecules. In other types of fermentation, such as ethanolic or heterolactic fermentation, the production of ethanol leads to other fermented foods and beverages such as sauerkraut, kimchi, and wine.
The production of lactic acid forms the basic structure and texture of yogurt. However, other molecules contribute to the taste of yogurt. These include acetaldehyde, an important flavor substance in yogurt, and tyrosine, a product of proteolytic activity, but can cause bitterness when the concentration is above 0.5 mg/ml (Guzel-Seydim, Sezgin, & Seydim, 2005).
Yogurt Production
Yogurt production can occur both on a large scale as well as at home for individual consumption (Fig. 4). As outlined by Cornell University’s Milk Quality Improvement Program (www.milkfacts.info), yogurt production begins by heating the milk to 85-90°C to kill any unwanted bacteria, such as those that can spoil milk or are pathogenic, as well as to denature the milk proteins so that they form more of a gel-like texture by holding in the moisture. This pasteurization step is important both for the consumer and the active cultures that will be added, since it eliminates potential competitors in the environment. Once the milk is cooled to around 42°C, the starter culture is added. These starter cultures most often include Lactobacillus delbrueckii subspecies bulgaricus (known simply as Lactobacillus bulgaricus until 1984 and referred as such from here on) (Fig. 5) and Streptococcus salivarius subsp. thermophilus (or more commonly Streptococcus thermophilus) (Fig. 6). In fact, these two species are the only cultures required under the Code of Federal Regulations to be present in what can be called "yogurt", though there can be a wide variation in the strain that is used. Along with the starter culture, probiotics may be added, common ones being Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacteria. The temperature of the milk is then maintained around 42°C until the pH reaches 4.5, a sign of sufficient lactic acid production. As the name Streptococcus thermophilus suggests, these bacteria are thermophiles that grow the best under elevated temperatures. Thus, the 42°C environment encourages the starter cultures to grow, while it inhibits the growth of non-thermophiles, such as pathogenic bacteria. Caution is needed, however, since L. bulgaricus and S. thermophilus may be thermophiles but are killed at temperatures higher than 55°C. Once a pH of 4.5 is reached, the yogurt is cooled to around 7°C to stop fermentation. It is worth noting however, that fermentation, though at a much slower rate, occurs even at a low temperature and therefore over-fermentation can occur and lead to excess lactic acid and dead bacteria, resulting in a sour, unpalatable yogurt. Thus, even though yogurt is already a product of fermentation, it can technically still spoil.
Benefits of Yogurt
The benefits of yogurt have been recognized even before microbes were discovered. The use of yogurt to treat body ailments are mentioned in the Bible, and scientists of the early ages like Hippocrates considered fermented milk to be a medicine, prescribing sour milk for curing stomach and intestinal disorders (Oberman, 1985 as cited in Lourens-Hattingh and Vilijoen, 2001). A scientific explanation for the beneficial effects of yogurt was first proposed by Eli Metchnikoff, a Russian bacteriologist at the beginning of the 20th century. Metchnikoff suggested that the lactobacilli in yogurt are responsible for the healthy and long lifespan of Bulgarian people. This led to the naming of one of the species in the starter culture as Lactobacillus bulgaricus (Lourens-Hattingh & Vilijoen, 2001).
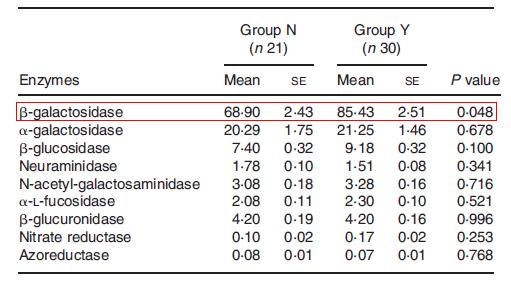
Since Metchnikoff’s time, advancements in technology have contributed to a better understanding of the benefits of yogurt consumption. In a study comparing regular yogurt consumers and non-consumers, it was found that L. bulgaricus was detected in 73% of fecal samples from consumers and 28% of fecal samples from non-consumers (Alvaro et al., 2007). Moreover, yogurt consumers had a significantly lower level of Enterobacteriaceae and higher activities of β-galactosidase (Fig. 7).
Within the yogurt-consumers, β-galactosidase activity and Bifidobacterium population were positively correlated with the amount of yogurt ingested. The researchers interpreted their findings as support for the beneficial effects of yogurt, since yogurt consumers had decreased levels of Enterobaceriaceae, which can include pathogens. Moreover, for lactose intolerant individuals, an increased activity of β-galactosidase by yogurt bacteria can aid in a quicker degradation of lactose. As it will be described below, Bifidobacteria and other probiotics are of particular interest in assessing the health benefits of yogurt.
Yogurt has also been found to protect against growth retardation in rats that are fed diets high in phytic acid, which disrupts zinc absorption, a mineral needed for normal growth (Gaetke et al., 2010). However, the level of zinc was low regardless of whether yogurt was added to the diet or not, suggesting that yogurt protects against growth retardation not by increasing zinc but by some other mechanism. Additionally, a variant of traditional yogurt, soy yogurt, has been found to help prevent hepatic lipid accumulation in rats (Kitawaki et al., 2009). Specifically, the rats fed soy yogurt had lower liver weight and hepatic triglyceride content, and their plasma cholesterol levels were also lower compared to control rats that were fed a standard rat diet without soy yogurt. Furthermore, ingestion of soy yogurt down-regulated the expression of sterol regulatory element binding protein (SREBP-1) and other lipogenic enzymes, while upregulating β-oxidation-related genes, that produce enzymes that are involved in the catabolism of fatty acids cholesterol in the rat liver.
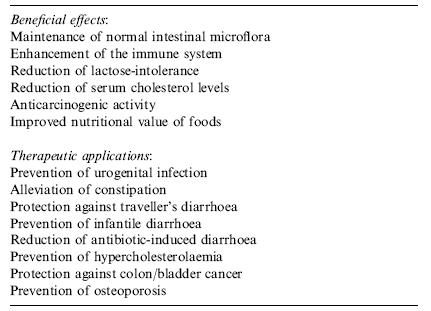
In general, there is a consensus that yogurt has beneficial effects for gastrointestinal health, as shown in both animal and human studies. Some studies also suggest that yogurt not only helps maintain a healthy gut, but can also help with certain gastrointestinal conditions, such as lactose intolerance, constipation, diarrheal diseases, colon cancer, Helicobacter pylori infection, inflammatory bowel disease, and allergies (Adolfsson, Meydani, & Russell, 2004). However, the bacteria responsible for these effects are not necessarily the bacteria that produced the yogurt, as discussed below.
Probiotics
The health benefits of yogurt for the most part can be directly attributed to probiotics (Fig. 8). Probiotics are defined as a mono- or mixed culture of live microorganisms which benefits the host by improving the host’s microflora (Lourens-Hattingh & Vilijoen, 2001).
Common probiotics added to yogurt are Lactobacillus casei (Fig. 9), Lactobacillus acidophilus,
and species of Bifidobacterium (Fig. 10).
These probiotics must be of a sufficient concentration in order to yield therapeutic effects of yogurt. It has been suggested that these bacteria should be present at a minimum level of 106 cfu/g in food products to confer the benefits to the consumer (Shah, 2000). A rather high concentration is needed to take into account organisms that may be eliminated during the passage through the gastrointestinal tract. Furthermore, yogurt is an effective vehicle for carrying probiotics because of its popularity as a ‘healthy’ food (Lourens-Hattings and Viljoen, 2001).
As mentioned above, the majority of beneficial effects of yogurt on gastrointestinal health are attributed to the probiotics. Thus for most cases, the benefits of yogurt are often the benefits provided by the probiotics, not just the fermented milk product. One particularly problematic condition of the GI tract is an infection caused by Helicobacter pylori. One study showed that the number of colonies decreased when H. pylori was inoculated with Bifidobacterium lactis and yogurt containing B. lactis and Lactobacillus acidophilus, though not with L. acidophilus alone (Fig. 11A).
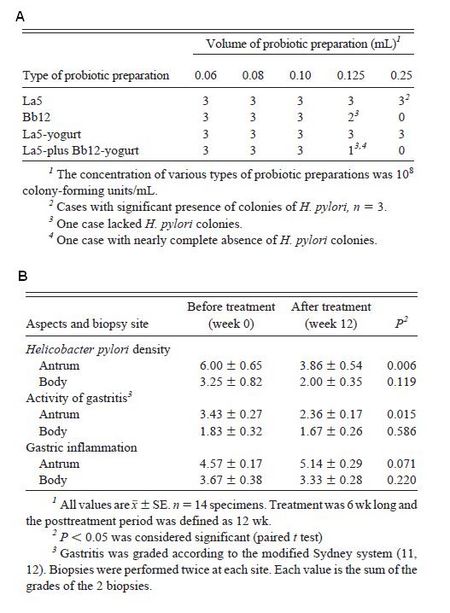
Moreover, when subjects with H. pylori infections were treated with a yogurt containing both B. lactis and L. acidophilus, biopsies showed that H. pylori density, activity of gastritis, and gastric inflammation were decreased, particularly in the antrum (Fig. 11B). This study showed the effect of probiotics on H. pylori survival and activity, providing robust evidence for the beneficial effects of probiotics.
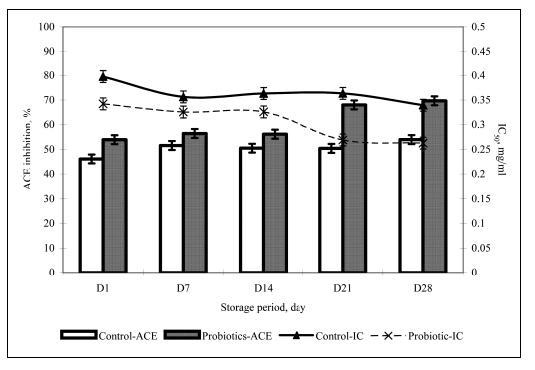
Probiotic cultures have also been found to be effective in soy yogurt as well. Donkor et al. (2005) reported that soy yogurt containing probiotics have higher activity inhibiting Angiotensin-converting enzyme (ACE). ACE is involved in the regulation of blood pressure, as it converts two types of angiotensins, which are highly potent vasoconstrictors, while simultaneously inactivating bradykinin, a vasodilatory peptide. Inhibiting this enzyme has been previously proposed as a method of attenuating hypertension (Nakamura et al., 1995 and others as cited in Donkor et al., 2005). Figure 12 shows that soy yogurt containing probiotic cultures inhibit ACE activity, supposedly just enough for antihypertensive effects but without achieving dangerously low vasoconstriction, possibly leading to low blood pressure. Studies such as these show the effect of probiotic cultures beyond the gastrointestinal area, supporting the claim that probiotics in yogurt may be even more beneficial than previously reported.
The designation of starter culture bacteria, L. bulgaricus and S. thermophilus, as probiotics is a topic of debate. The majority of the gastrointestinal benefits are attributed to the probiotics that are added after the yogurt is produced by the starter culture. However, some argue that the starter culture bacteria should also be called probiotics because they improve lactose digestion and eliminate symptoms of lactose intolerance when consumed in yogurt without any added probiotics (Guarner et al., 2005). In the rat colon, it has been found that the starter culture bacteria inactivate carcinogens, thus avoiding carcinogen-induced lesions, and also prevent DNA damage, which in effect prevents tumors (Wollowski et al., 1999). In summary, there is some research implicating that the starter cultures do confer some benefits that may deem them to be classified as probiotics, but it may take a while before this idea is accepted by the majority.
Improving Yogurt
Current Problems
Though the benefits of yogurt are recognized, what is not really understood is if the functionality of yogurt is at its maximum. The most obvious and heavily researched area is increasing the viability of the probiotics that provide the health benefits. However, increasing viability extends to the starter cultures as well, as they have also been documented to contribute some benefits and more importantly, need to be alive in order to be able to produce yogurt even in the presence of other chemicals. Viable cell count/density is the reason not all yogurt brands are equal. Those with live active cultures are indeed healthier than the pasteurized version in which the yogurt is heated, thus killing any live bacteria. However, some brands of yogurt have a higher concentration of live cultures compared to others, though for marketability reasons and overall difficulty in assessing live culture concentration, these numbers are often undisclosed. Improving yogurt by increasing or enhancing bacterial viability is a large field on its own, and is thus discussed below.
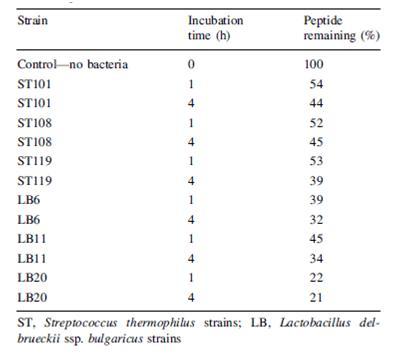
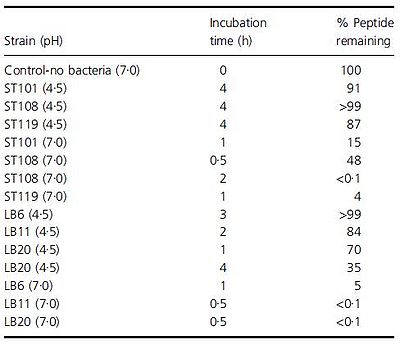
Another problem that is not as noticed but certainly plausible is the breakdown of beneficial molecules by the bacteria in yogurt. Paul and Somkuti (2009, 2010) have looked at the degradation of milk-derived peptides that are beneficial to humans. One such peptide is lactoferricin, a 25-amino acid antimicrobial peptide, also reported to have immunostimulatory, antioxidative, and anticarinogenic properties (Paul & Smokuti, 2010). Lactoferricin is released by the pepsin digestion of lactoferrin found in milk. However, the researchers found that when incubated with various strains of S. thermophilus or L. bulgaricus, the amount of lactoferricin decreased over time, suggesting that these species are breaking it down (Fig. 13). Though these results raise the question of how one can get around the hydrolytic breakdown of this peptide with such high potential, it is also worth noting that this particular experiment was carried out only at pH 7. The pH of yogurt is certainly lower, around 4.6, and thus a pH 7 is not representative of the conditions that lactoferricin would be found along with S. thermophilus and L. bulgaricus.
An earlier paper by Paul and Smokuti (2009) looked at the effect of pH on hydrolytic breakdown of certain peptides. Though this experiment focused on different peptides, specifically, an 11-mer antimicrobial and 12-mer hypotensive peptides, they found that the peptides are degraded a lot more quickly at pH 7 (Fig. 14). In fact, at pH 4.5 for some of the strains, over 90% of the original peptide is intact even after incubating for 4 hours. The researchers suggest that while peptides found in the milk at the beginning of yogurt production may be degraded, as the pH of milk is around 6.7-6.8 (www.milkfacts.info), if the peptides are added after yogurt is produced, the peptides may avoid degradation by the bacteria since the pH would have dropped. Thus, the benefits of these antimicrobial and hypotensive peptides may be reaped if they are added at the right time during yogurt production so that they are not broken down before they can be consumed.
Improving functionality of Yogurt
As mentioned above, research today focuses on methods of increasing viability of the live cultures, particularly the probiotics, as they are responsible for conferring the health benefits of yogurt. Perhaps one of the most basic ways to manipulate probiotic viability is by altering environmental conditions and specific starter cultures to find an optimal combination. Abe et al. (2009) manipulated temperature, both for fermentation and storage, and the type of starter culture used (frozen vs. lyophilized, only Bifidobacteria vs. Bifidobacteria along with the presence of Lactococcus lactis ). Combining all of these factors, the researchers concluded that Bifidobacteria are able to survive in yogurt, but are mostly affected by the fermentation temperature and bacterial composition of the starter when they are refrigerated while in storage (Abe et al., 2009). In another study, the type of milk was the variable, where the researchers studied probiotic growth in soy milk compared to cows' milk, the ingredient of standard yogurt (Farnworth et al., 2007). The study reports that while probiotics can grow in soy yogurt, they grow slower and the bacteria utilize different sugars to support their growth compared to cows' milk yogurt.
In addition to manipulating common variables like temperature and starter culture composition, Shah (2000) suggests various methods by which probiotic viability can be increased. First, in order to have an accurate assessment of viability count, he recommends the development of selective enumeration techniques, such as using a media that enhances a specific bacterial species to grow and incubating at an optimal temperature and pH. As for establishing viability, one must pay attention to the combination of organisms present in the yogurt, since there could be antagonistic relationships, as well as to growth requirements and oxygen levels, as some species like Bifidobacteria are anaerobic and are susceptible to oxygen toxicity. Moreover, in order to improve viability, Shah (2000) suggests some techniques like selecting acid and bile resistant strains, using glass containers over plastic containers to let the milk ferment, using a sufficient concentration of inoculum, and adding micronutrients to enhance growth. Other factors suggested by Lourens-Hattings and Viljoen (2001) include the interaction between the species present, chemical composition of the fermentation medium, such as carbohydrate source, final acidity, milk solids content, and availability of nutrients and growth promoters/inhibitors.
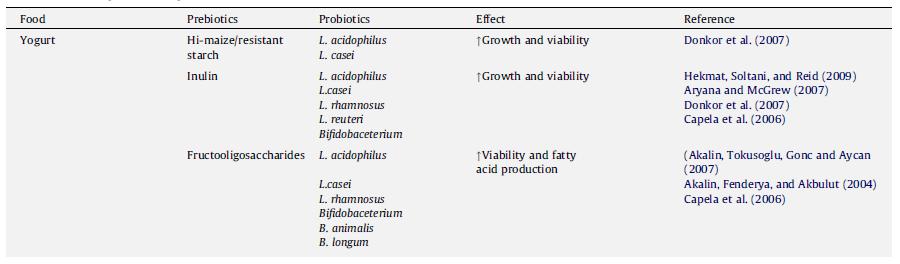
In a sense, any factor manipulated in yogurt production (besides altering the genes of the bacteria) is essentially changing the environment, since even the addition of chemicals or other species alters the environment by introducing the presence of a new nutrient or competitor. Many studies have looked at the effect of adding a chemical or molecule on probiotic viability. A group of molecules that has received special attention is the prebiotics. Defined as “a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon” (Roberfroid, 2007), prebiotics are particularly popular because they are derived from food, and thus one does not need to worry about potential harmful effects since they are regularly consumed anyway. A summary of some of the common prebiotics and its effects on probiotics are shown in Figure 15.
When both prebiotics and probiotics are found in yogurt, it is considered to be a synbiotic, since there is a synergistic effect where the prebiotic enhances the benefits of the probiotic organisms (Schrezenmeir & Vrese, 2001). In addition to prebiotics like inulin, which increases the activity of Lactobacillus acidophilus, increases calcium absorption, and is a good source of dietary fiber (Aryana et al., 2007), and fructooligosaccharides (Akalin et al., 2007), which can be used to replace fat, other compounds have been added to yogurt. These include chitosan, the main derivative of chitin and thought to have hypoglycemic effects (Seo et al., 2009), Versagel©, a whey-protein based fat replacer (Ramchandran & Shah, 2008) and β-glucan, another prebiotic that is just beginning to receive more attention (Vasiljevic, Kealy, & Mishra, 2007). Besides compounds and chemicals, other bacterial species, such as Spirulina platensis, have been added to yogurt to see how it would interact with probiotic bacteria and affect their viability (Akalin, Unal, & Dalay, 2009). However, moderation is also of importance, as an excessively high inoculation level of L. acidophilus (2.33g/100g) resulted in an inferior quality yogurt. Evaluators rated this yogurt as tasting weak and having a gel-like, ropy texture with lots of free why floating around. Yogurt with such a high concentration of L. acidophilus was found to have much lower lactobacilli counts, most likely because the L. acidophilus outcompeted L. bulgaricus and other species (Olson & Aryana, 2008). Thus, the weak taste associated with this yogurt may be attributable to the lower concentration of L. bulgaricus in the yogurt.
Towards a "Superior" Yogurt
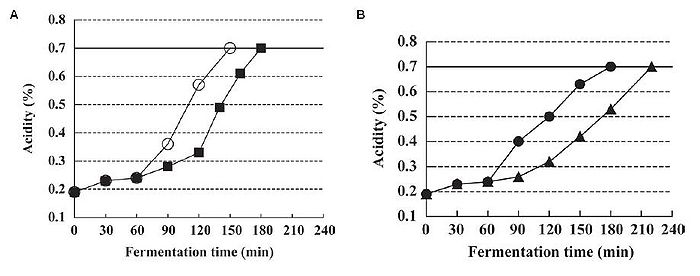
In addition to improving the health potential of yogurt, from an industrial point of view, there is interest in manufacturing a more palatable type of yogurt that appeals to the masses and can be produced efficiently. Such yogurt is generally smooth, mild, and pleasantly sour, though these characteristics may be subject to individual taste. The outcome of yogurt is affected by the quantity and rate of lactic acid addition. L. bulgaricus and S. thermophilus are known to be facultative anaerobic bacteria that can grow in oxygenated environments. It has been found that these species remove the dissolved oxygen in the yogurt mix during fermentation, and only actively begin to produce lactic acid after the dissolved oxygen (DO) concentration in the yogurt mix is lowered to 0 mg/kg (Horiuchi et al., 2009). This suggests that lactic acid production is suppressed by dissolved oxygen in the yogurt mix. By altering the DO concentration to begin with, one could essentially control when lactic acid production begins. One example of such control is by reducing the dissolved oxygen concentration. Since DO suppresses lactic acid production, reducing DO negates this suppression, allowing lactic acid fermentation to begin. This process is known as reduced dissolved oxygen fermentation (ROF). In addition to jumpstarting lactic acid production, ROF also prolonged the lactic acid production process in a culture that started with 0 mg/kg DO, compared to the control of around 6 mg/kg DO. This means that it takes less time to reach a desired acidity level if yogurt is produced by ROF, as one does not need to wait for the dissolved oxygen to be depleted before fermentation can start (Fig. 16A). Moreover, despite initiating lactic acid production much earlier and maintaining it for a longer time, the viable cell counts of the bacterial species and characteristics of the ROF yogurt such as acidity and curd tension were no different from the control yogurt (Horiuchi et al., 2009). One advantage of using ROF is the reduction of production time, as the cultures enter the exponential growth phase sooner.
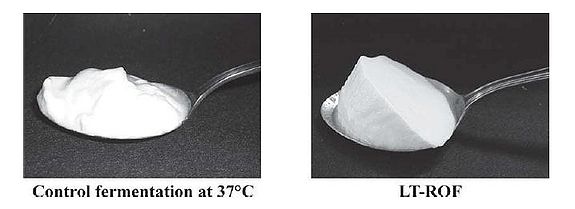
It has been shown that yogurt made at a low temperature produces smooth yogurt and the physical properties of yogurt are improved as the starter culture are given more time to produce aroma substances and other accessory molecules that affect the taste, and are able to block fast acid production (Guzel-Seydim, Sezgin, & Seydim, 2005). Given this fact, Horiuchi et al. (2009) set out to make a yogurt that was smooth but could be counteracted with ROF to reduce the wait time compared to traditional low temperature production, which requires more time in exchange for a smooth product. Using low temperature reduced dissolved oxygen fermentation (LT-ROF), the researchers achieved a "superior" set yogurt with a smooth texture and a strong curd structure. Moreover, the marketability of this type of yogurt production is increased, as Horiuchi et al. (2009) have shown that yogurt produced by LT-ROF takes less time than traditional yogurt produced at 37°C (Fig. 16B), and is comparable to the faster common yogurt production that takes place at 43°C (compare control of Fig. 16A and LT-ROF of Fig. 16B).
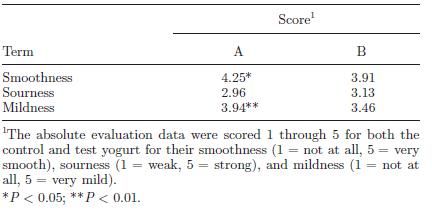
Regardless of the actual percentage of acidity, what is important is how the yogurt appeals to consumers. The LT-ROF yogurt was evaluated to be smoother and milder on average by 200 consumers compared to control yogurt produced at the standard temperature of 43°C (Fig. 17). However, the LT-ROF yogurt turned out to have a smooth texture comparable to the control yogurt made at a lower temperature (37°C). The major difference between these two types of yogurt was the firmness of the curd (Fig. 18). Horiuchi et al. (2009) suggested that having a firm curd was beneficial since manufactured yogurt needs to be transported in trucks, thereby requiring a firmer texture that can withstand the shaking. The researchers thus concluded that the LT-ROW method of producing yogurt was the superior method as it takes about the same time as producing yogurt at 43°C and results in a smooth yogurt that can hold its shape while in transit.
Another study focused on the effect of temperature and starter culture type on the quality of yogurt. Guzel-Seydim, Sezgin, and Seydim (2005) compared how the quality of yogurt changes based on whether it is produced at high (45°C) or low temperatures (35°C) and whether exopolysaccharide-producing or non-producing strains are used. These exopolysaccharides are of interest because it is a ropy, mucoid substance that increases the viscosity of yogurt and decreases whey separation. Guzel-Seydim et al. (2005) quantified the quality of yogurt by looking at the pH, lactic acid percentage, total volatile fatty acids content, acetaldehyde content, tyrosine content, consistency, viscosity, and extent of whey separation of the yogurt samples. They found that the ropy exopolysaccharide-producing strains had a better texture overall when incubated at the lower temperature, but the non-exopolysaccharide strains actually had a better taste, as evaluated by the higher acetaldehyde content. Nonetheless, the researchers suggest that these exopolysaccharide-producing strains may be useful in replacing additives like fat that are used to improve the texture of yogurt. Thus, as is the case with most foods, one would hope to improve not just the taste and texture of yogurt, but also the nutritional value, which also ties back to the health benefits that were described earlier.
Conclusion
Yogurt has a long history and its benefits have been valued by many people, particularly those with gastrointestinal problems. The production behind yogurt is well understood, allowing for improvements and advancements in both the quality and efficient manufacturing of the product. Improving the health potential of yogurt has become a popular field, and for industrial reasons, enhancing the taste and texture, as well as storage life of yogurt is an appealing advancement for yogurt consumers. Yogurt in its basic form is a very eco-friendly product, as humans are essentially consuming the waste products of acidic fermentation. Additionally, the unique taste, texture, and potential for even better health benefits make yogurt an attractive food for people of many cultures.
References
Akalin, A. S., G. Unal., & M. C. Dalay. 2009. Influence of Spirulina platensis biomass on microbiological viability in traditional and probiotic yogurts during refrigerated storage. Ital. J. Food Sci. 21: 356-364.
Olson, D. W. & K. J. Aryana. 2008. An excessively high Lactobacillus acidophilus inoculation level in yogurt lowers product quality during storage. LWT-Food Sci. and Tech., 41: 911-918. Paul, M. and G. A. Somkuti. 2009. Degradation of milk-based bioactive peptides by yogurt fermentation bacteria. Lett Appl Microbiol. 49:345–350.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2010, Kenyon College.