The Role of Endogenous Retroviruses in Placental Gene Expression: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
===Placental & Endogenous Retrovirus Structural Components=== | ===Placental & Endogenous Retrovirus Structural Components=== | ||
[[Image: Fig 1-Endogenic Retrovirus.jpeg|thumb|500px|left|Figure 1:</b> The General Structure of an ERV ( | [[Image: Fig 1-Endogenic Retrovirus.jpeg|thumb|500px|left|Figure 1:</b> (A) The General Structure of an ERV with (B) Core Encoded Proteins, and (C) a lone Long Terminal Repeat (LTR) (characteristic of <i>env</i> genes).<ref name = Soygur2016>[Soygur, B., & Sati, L. (2016). The role of Syncytin proteins in human reproduction and reproductive organ cancers, Reproduction, 152(5), R167-R178. Retrieved Apr 4, 2021, from https://rep.bioscientifica.com/view/journals/rep/152/5/R167.xml]</ref>]] | ||
The presence of ERVs in host genomes is the result of either direct insertion by host homologous end joining, or from transposition through the use of retrotransposons.<ref name = Lavialle2013/> Approximately 8% of the human genome consists of endogenous retroviral elements.<ref>[Griffiths, D. J. (2001). Endogenous retroviruses in the human genome sequence. <i>Genome biology,</i> 2(6), 1-5. ]</ref> Although exogenous retroviruses and ERVs have different impacts on the human body, they share the same structure which consists of four viral genes: gag, pro, pol and env, flanked by two long terminal repeats (<b>Figure 1</b>). Gag genes encode for major viral structural proteins; pro and pol encode for viral enzymatic machinery and Env encodes the envelope glycoprotein. The envelope glycoprotein embeds into the lipid bilayer of the exterior membrane in order to form the viral envelope and controls the entry of viruses into susceptible cells.<ref name = Black2010>[Black, S. G., Arnaud, F., Palmarini, M., & Spencer, T. E. (2010). Endogenous retroviruses in trophoblast differentiation and placental development. <i>American journal of reproductive immunology (New York, N.Y. : 1989), 64(4),</i> 255–264. https://doi.org/10.1111/j.1600-0897.2010.00860.x ]</ref> These components of the envelope protein are significant in the way which ERV evolution occurred in mammals and the way in which envelope genes can affect the different functional components of mechanisms within the placenta. The placenta itself has several key structural components which are important to understand. | The presence of ERVs in host genomes is the result of either direct insertion by host homologous end joining, or from transposition through the use of retrotransposons.<ref name = Lavialle2013/> Approximately 8% of the human genome consists of endogenous retroviral elements.<ref>[Griffiths, D. J. (2001). Endogenous retroviruses in the human genome sequence. <i>Genome biology,</i> 2(6), 1-5. ]</ref> Although exogenous retroviruses and ERVs have different impacts on the human body, they share the same structure which consists of four viral genes: gag, pro, pol and env, flanked by two long terminal repeats (<b>Figure 1</b>). Gag genes encode for major viral structural proteins; pro and pol encode for viral enzymatic machinery and Env encodes the envelope glycoprotein. The envelope glycoprotein embeds into the lipid bilayer of the exterior membrane in order to form the viral envelope and controls the entry of viruses into susceptible cells.<ref name = Black2010>[Black, S. G., Arnaud, F., Palmarini, M., & Spencer, T. E. (2010). Endogenous retroviruses in trophoblast differentiation and placental development. <i>American journal of reproductive immunology (New York, N.Y. : 1989), 64(4),</i> 255–264. https://doi.org/10.1111/j.1600-0897.2010.00860.x ]</ref> These components of the envelope protein are significant in the way which ERV evolution occurred in mammals and the way in which envelope genes can affect the different functional components of mechanisms within the placenta. The placenta itself has several key structural components which are important to understand. | ||
| Line 17: | Line 17: | ||
===The Surface and Transmembrane Subunits=== | ===The Surface and Transmembrane Subunits=== | ||
[[Image: Endogenousenvelope.jpg|thumb|400px|right|Figure 2:</b> The | [[Image: Endogenousenvelope.jpg|thumb|400px|right|Figure 2:</b> The Retroviral Envelope Glycoprotein. (a) Structure of retroviral envelope protein with TM and SU subunit. (b) Interaction between a retroviral envelope protein and its cognate receptor: i) Virion-cell membrane fusion and virus entry into a specific cell and ii) Cell-cell membrane fusion and formation of a synctium.<ref name = Lavialle2013/>]] | ||
The different components of the retroviral envelope consists of the surface (SU) and transmembrane (TM) subunits as well as the immunosuppressive domain (ISD).<ref name=Lavialle2013/> The env gene of retroviruses encodes for a protein which is cleaved by a cellular protease in order to produce the TM and SU subunits. The SU subunit has a domain which interacts with a receptor on the surface of cells that is only susceptible with the virus. The TM subunit is responsible for triggering the fusion of the virus and cell membranes by insertion of its N-terminal fusion peptide into the cell membrane.<ref name=Lavialle2013/> These subunits remain as a heterodimer at the surface of the virion envelope membrane (<b>Figure 2</b>). The role of these subunits is important with respect to the immunosuppressive activity of the Syncytin-2 protein. | The different components of the retroviral envelope consists of the surface (SU) and transmembrane (TM) subunits as well as the immunosuppressive domain (ISD).<ref name=Lavialle2013/> The env gene of retroviruses encodes for a protein which is cleaved by a cellular protease in order to produce the TM and SU subunits. The SU subunit has a domain which interacts with a receptor on the surface of cells that is only susceptible with the virus. The TM subunit is responsible for triggering the fusion of the virus and cell membranes by insertion of its N-terminal fusion peptide into the cell membrane.<ref name=Lavialle2013/> These subunits remain as a heterodimer at the surface of the virion envelope membrane (<b>Figure 2</b>). The role of these subunits is important with respect to the immunosuppressive activity of the Syncytin-2 protein. | ||
Revision as of 04:14, 7 April 2021
By Anna Harnsberger
Introduction
The role of integrated or “endogenous” retroviruses (ERVs) is extensive. ERVs contribute both positively and negatively toward the health of humans and other taxonomic groups. The functionality of these viruses relies heavily on the process of retrotransposition. This process involves the reverse-transcription of the retrovirus genome into the DNA of the host cell. Though some ERVs are detrimental to human health, such as HIV-1, others are “domesticated” and do not pose a threat.[1] Domesticated ERVs, likely inactivated by mutations over millions of years, serve in adaptive functions of the host. [2] Specifically, the presence of ERVs are essential during human pregnancy. The placenta, a vital organ developed during gestation, is affected by the presence of ERVs. ERVs, acting as non-coding regulatory elements, are key contributors to placental evolution. Absence or mis-expression of specific ERVs in the placenta during pregnancy, results in pre- and or post- defects such as pre-eclampsia (PE). Despite what is known about the importance of ERVs in placental gene expression, there is still much research to be done to fully understand the impacts of these retroviruses on the placenta.
The Role of Endogenous Viruses in the Placenta
ERVs are an essential component of the development of the placenta, not only for humans but a vast array of animals. The origin of ERVs into the placenta is understood to have occured approximately 100 million years ago (Myr). ERVs parasitized living species even before the viruses began to appear in mammals.[3] As opposed to exogenous viruses which are associated with infection, ERVs are germ-line integrated into the host DNA by vertical gene transfer.[4] ERVs evolve more slowly than their exogenous counterparts and function in a sort of symbiosis with their host, given their normal presence in the genome. The importance of ERVs in the placenta focuses on specific envelope genes that originate from the retrovirus, these will be covered in depth in the following sections. The core components to consider when understanding the importance of ERVs is their role in cell differentiation, cell fusion and their immunosuppressive properties.
Placental & Endogenous Retrovirus Structural Components
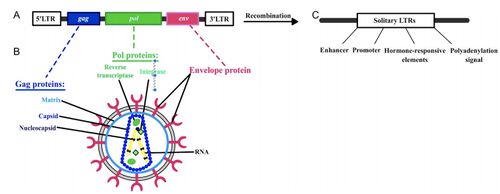
The presence of ERVs in host genomes is the result of either direct insertion by host homologous end joining, or from transposition through the use of retrotransposons.[3] Approximately 8% of the human genome consists of endogenous retroviral elements.[6] Although exogenous retroviruses and ERVs have different impacts on the human body, they share the same structure which consists of four viral genes: gag, pro, pol and env, flanked by two long terminal repeats (Figure 1). Gag genes encode for major viral structural proteins; pro and pol encode for viral enzymatic machinery and Env encodes the envelope glycoprotein. The envelope glycoprotein embeds into the lipid bilayer of the exterior membrane in order to form the viral envelope and controls the entry of viruses into susceptible cells.[7] These components of the envelope protein are significant in the way which ERV evolution occurred in mammals and the way in which envelope genes can affect the different functional components of mechanisms within the placenta. The placenta itself has several key structural components which are important to understand.
Nomenclature to understand with respect to this topic includes the placental tissue, also known as the trophoblast, composed of peripheral cells of the blastocyst. The blastocyst is a specific stage of the mammalian embryo, with a composition similar to that of a hollow cell ball. Furthermore, cytotrophoblasts are important for attachment to the inner uterine wall. These cells are important for cell-cell fusion along with myoblasts, macrophages, and gametes. Most notably, cytotrophoblasts are important in formation of syncytiotrophoblasts. Syncytiotrophoblasts aid in mediating implantation of the embryo into the maternal endometrium as well as functions of exchange related to the mother and fetus, regulatory immune response, protection against pathogens and hormone secretions.[3] The terminology related to placental development is necessary to understand, with respect to how ERVs affect the organ.
The Surface and Transmembrane Subunits
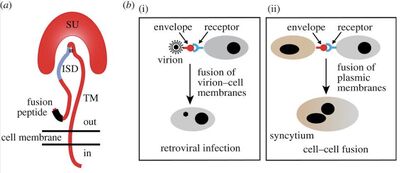
The different components of the retroviral envelope consists of the surface (SU) and transmembrane (TM) subunits as well as the immunosuppressive domain (ISD).[3] The env gene of retroviruses encodes for a protein which is cleaved by a cellular protease in order to produce the TM and SU subunits. The SU subunit has a domain which interacts with a receptor on the surface of cells that is only susceptible with the virus. The TM subunit is responsible for triggering the fusion of the virus and cell membranes by insertion of its N-terminal fusion peptide into the cell membrane.[3] These subunits remain as a heterodimer at the surface of the virion envelope membrane (Figure 2). The role of these subunits is important with respect to the immunosuppressive activity of the Syncytin-2 protein.
The Evolutionary Diversification of the Placenta
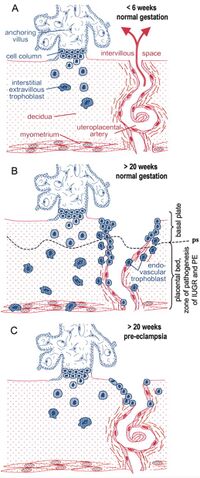
Despite the emergence of ERVs in the placenta after the evolution of mammals, there are multiple factors which lead to differentiation of the placenta amongst these different species. Including diversity of the cytotrophoblasts, syncytiotrophoblasts and the degree of invasiveness of trophoblasts. Amongst different species, there is variation of the types of placental interface. There is an increase of invasive trophoblasts from synepitheliochorial (ruminants), endothelialcorial (carnivores), and hemochorial (mice, rabbits, humans). Trophoblast invasion is important in all of these species, since malinvasion can result in PE or Intrauterine growth retardation (IUGR). (Figure 3)[8]
Synctin: Key Proteins Originating from Endogenous Retroviruses
When considering the role of ERVs on the development and regulation of the placenta, the key proteins that should be considered are “Synctins. Synctins are cellular proteins which correspond to functional ERV env open reading frames as single copies, which have been conserved throughout evolution.[9] Syncytin proteins play an important role in cell-cell fusogenic activity and immunosuppression of the envelope proteins. [3]
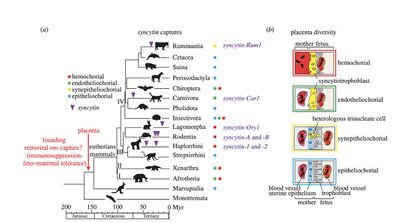
There are two notable types of Syncytin proteins found in the human placenta, encoded by Env genes HERV-W (Syncytin-1) and HERV-FRD (Syncytin-2). Syncytin-1 is a glycoprotein, with cell fusogenic activity, promoting the fusion of cytotrophoblast cells in order to form the multinucleated syncytiotrophoblast layer. Syncytin-2 is associated in its role of immunosuppression.[5] The membrane receptor for Syncytin-1 is ASCT-2 and ASCT-1 and the phospholipid transporter MFSD-2 for Syncytin-2.[9] The functionality of these two genes has been conserved in evolution since their first insertion into the primate genome, approximately 25-40 Myr, Syncytin-2 is the more ancient of the two genes. Both genes have little polymorphism and therefore show signs of purifying selection or stabilizing selection. [3] Analogous to the human “Syncytin-1” is the muridae gene “Synctin-A”, the human “Synctin-2” gene is analogous to the muridae “Synctin-B” gene. It is important to note that although the muridae and human Syncytin proteins are similar in their functions, they are homologous but not orthologous(Figure 4).[3]
Fusogenicity of Syncytin-A and Syncytin-1
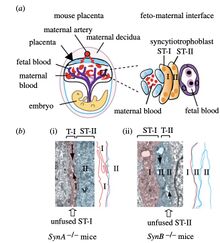
Given the likeness of the muridae Syncytin proteins with those of humans, muridae have been a beneficial model for determining the role that Syncytin proteins play in placental development. It has been found that using the mouse model, that synctin-B and Gcm1 (the placental transcription factor) genes are both expressed in the ST-II layer, one of the two distinct layers of syncytiotrophoblasts within mice. [3] The two ST-layers arise from the fusion of trophoblast cells during the “labyrinth formation”(Figure 5).[3] The labyrinth is the interface for nutrient and other gas exchange between the embryo and mother.[10] The ST-II layer contains an abundance of lipids which are tightly adhered to one another through gap junctions available for intracellular transport.[11]
References
- ↑ [Haig, D. (2012). Retroviruses and the placenta. Current Biology,22(15), R609-R613.]
- ↑ [Knox, K., & Baker, J. C. (2008). Genomic evolution of the placenta using co-option and duplication and divergence. Genome research, 18(5), 695-705. ]
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 [Lavialle, C., Cornelis, G., Dupressoir, A., Esnault, C., Heidmann, O., Vernochet, C., & Heidmann, T. (2013). Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1626), 20120507. ]
- ↑ [Harris J. R. (1998). Placental endogenous retrovirus (ERV): structural, functional, and evolutionary significance. BioEssays : news and reviews in molecular, cellular and developmental biology, 20(4), 307–316.]
- ↑ 5.0 5.1 [Soygur, B., & Sati, L. (2016). The role of Syncytin proteins in human reproduction and reproductive organ cancers, Reproduction, 152(5), R167-R178. Retrieved Apr 4, 2021, from https://rep.bioscientifica.com/view/journals/rep/152/5/R167.xml]
- ↑ [Griffiths, D. J. (2001). Endogenous retroviruses in the human genome sequence. Genome biology, 2(6), 1-5. ]
- ↑ [Black, S. G., Arnaud, F., Palmarini, M., & Spencer, T. E. (2010). Endogenous retroviruses in trophoblast differentiation and placental development. American journal of reproductive immunology (New York, N.Y. : 1989), 64(4), 255–264. https://doi.org/10.1111/j.1600-0897.2010.00860.x ]
- ↑ 8.0 8.1 [Kaufmann, P., Black, S., & Huppertz, B. (2003). Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biology of reproduction, 69(1), 1-7. ]
- ↑ 9.0 9.1 [Coquin, Y., Ferrand, M., Seye, A., Menu, L., & Galy, A. (2019). Syncytins enable novel possibilities to transduce human or mouse primary B cells and to achieve well-tolerated in vivo gene transfer. bioRxiv, 816223. ]
- ↑ [Tai-Nagara, I., Yoshikawa, Y., Numata, N., Ando, T., Okabe, K., Sugiura, Y., Ieda, M., Takakura, N., Nakagawa, O., Zhou, B., Okabayashi, K., Suematsu, M., Kitagawa, Y., Bastmeyer, M., Sato, K., Klein, R., Navankasattusas, S., Li, D. Y., Yamagishi, S., & Kubota, Y. (2017). Placental labyrinth formation in mice requires endothelial FLRT2/UNC5B signaling. Development (Cambridge, England), 144(13), 2392–2401.https://doi.org/10.1242/dev.149757]
- ↑ [Dupressoir, A., Vernochet, C., Bawa, O., Harper, F., Pierron, G., Opolon, P., & Heidmann, T. (2009). Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proceedings of the National Academy of Sciences, 106(29), 12127-12132. ]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2021, Kenyon College.
