The Temperature Relationship of Batrachochytrium dendrobatidis
Introduction
By [Eva Brazer]
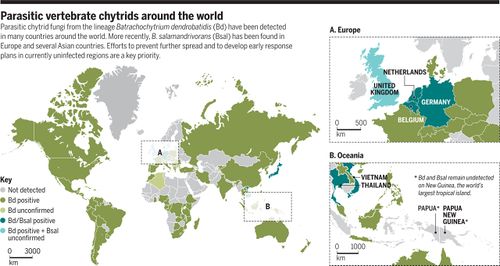
Amphibian species around the world are experiencing unprecedented population decline due to the emerging infectious disease chytridiomycosis, which is caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd)[2]. The chytrid pathogen is considered an emerging infectious disease because it was discovered and described only in the last twenty years[3], and has continued to spread globally causing devastating effects[4]. Batrachochytrium dendrobatidis has been identified on every continent on which amphibians are present[2].
Recent global mapping initiatives reported that Batrachochytrium dendrobatidis had been identified as having infected 1,015 of the 1,854 species examined (54%). Further population analysis found that the fungus has resulted in the decline of at least 501 species (6.5% of all amphibian species), with 124 experiencing a decrease in the species population of over 90%[5]. Over a hundred presumed extinctions have been attributed to chytridiomycosis[6]. The catastrophic impact of Batrachochytrium dendrobatidis is so significant that it is believed to be the largest threat to the biodiversity of any class of vertebrate[2]. Batrachochytrium dendrobatidis has been documented in hundreds of amphibian species, and reports of infection in new species and geographic locations are continually increasing[6].
Climate change models are predicting an increase in the frequency and magnitude of temperature fluctuations[7]. This has caused concern that these temperature fluctuations may lead to increased pathogen development, transmission, and ectotherm-host susceptibility[8]. The parasitic performance of Batrachochytrium dendrobatidis is sensitive to temperature[8]. By examining the relationship between pathogen and host interactions particularly in relation to temperature fluctuations, scientists can better predict and mitigate the spread of Batrachochytrium dendrobatidis to new amphibian populations.
Phylogeny
Scientific classification
Kingdom: Fungi
Division: Chytridiomycota
Class: Chytridiomycetes
Order: Rhizophydiales
Genus: Batrachochytrium
Species: B. dendrobatidis
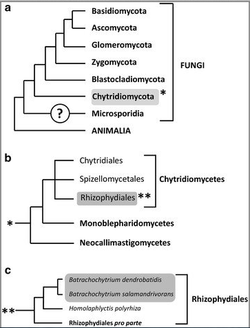
After its discovery, the amphibian chytrid fungus was placed in a new genus, Batrachochytrium, which was originally in the order Chytridiales[10]. The designation of the fungus was initially determined by examining the zoospore ultrastructure[1]. However the discovery of new ultrastructural and molecular characteristics helped to segregate different orders, designating Batrachochytrium dendrobatidis as a member of the order Rhizophydiales[11]. This separation was supported by analysis of the small subunit ribosomal DNA (ssu-rDNA) sequence of the pathogen. At the time of its designation, Batrachochytrium dendrobatidis was the only known member of its genus[10]. It was also the only known member of Chytridiomycota to parasitize vertebrates[6], and is one of a very limited number of endobiotic fungi in the order Rhizophydiales[12].
It was not until 2013 that another member of the Batrachochytrium genus became known. A second pathogenic species, Batrachochytrium salamandrivorans (Bsal) was discovered[5]. Similarly to B. dendrobatidis, B. salamandrivorans causes chytridiomycosis and death in its host, however in this case the species preferentially infects salamanders[13].
Infection of Host
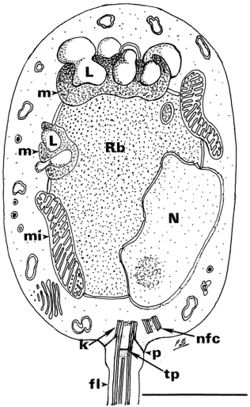
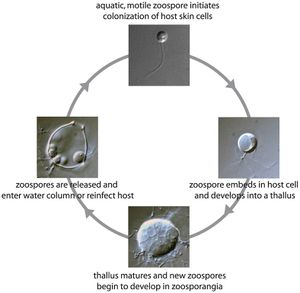
Chytridiomycosis is caused by an aquatic flagellated fungus that targets amphibian hosts[6]. Batrachochytrium dendrobatidis colonizes the superficial epidermis, resulting in a thickening of the outer layer of skin. While cutaneous fungal infections like chytridiomycosis are rarely fatal in vertebrate species, amphibians are particularly susceptible due to the unique physiological qualities of their skin[1]. In amphibians, the skin plays a crucial role in regulating the exchange of respiratory gases, water and electrolytes. By disrupting the epidermal layer, Batrachochytrium dendrobatidis can cause complications in osmoregulation, hydration, breathing, and thermoregulation[6]. In susceptible species, death usually occurs between 18 and 70 days after initial exposure. The incubation time varies with species, amount of exposure and temperature[1]. Relatively resistant amphibian species that show no clinical signs often still carry light infections and act as asymptomatic carriers, spreading the disease.
In tadpoles, keratin is localized to the mouthparts, however after metamorphosis the skin of the mature amphibian becomes keratinized. Because Batrachochytrium dendrobatidis targets keratinized areas of the organism, the detrimental impact of the fungal infection is more pronounced in the post-metamorphic stages, often resulting in death. In tadpoles the localization of infection to the mouthparts often results in sublethal effects. However, damage to the mouthparts can limit nutrient uptake and impair growth[1][2].
In amphibians, clinical signs of chytridiomycosis at the cellular level include, damaged nuclei, altered cytoplasm and most significantly a hyperplastic response[14], a very common response to epidermal injury or infection[1]. Hyperplasia causes excessive skin sloughing due to increased turnover of epidermal cells and swelling of the cells nearest the foci of infection[14]. This abnormal molting is significant because it can be an indication of the mortality risk for chytridiomycosis. If the species has thick skin and therefore a sufficient supply of epidermal layers, epidermal cell turnover can increase to rid the amphibian of all infected cells[12]. However, thinning of the epidermis may occur if epidermal cells are not regenerated at a rate consistent with the rapid rate at which cells are sloughing from the surface, conversely if the rate of regeneration exceeds the rate of cell turnover, buildup can occur, impeding cutaneous functions[6]. If the disruption of normal physiological processes in the epidermis is significant enough, the resulting osmotic imbalance and electrolyte depletion can be fatal[12]. The interference of the fungus with ion transport functions in the epidermis eventually results in cardiac arrest and death[15]. At the macroscopic level, signs like lethargy, anorexia, loss of reflexes and sometimes-abnormal posture is observed, however this is generally only observed immediately before death[12].
Life Cycle
Effect of Temperature
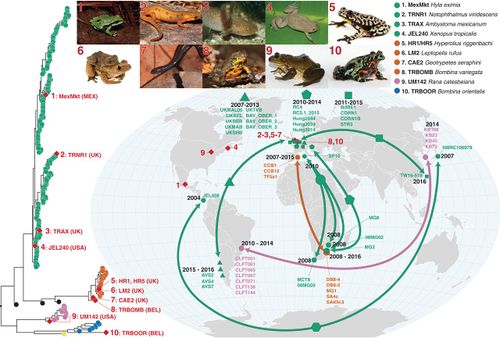
Geographic Distribution
Effect of Temperature
Climate Change
Conclusion
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Berger et al. 2005. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Inter-Research. 68:52-63
- ↑ 2.0 2.1 2.2 2.3 Weldon et al. 2004. Origin of the Amphibian Chytrid Fungus. Emerging Infectious Diseases. 10(12):2100-2105
- ↑ Berger et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. PNAS. 95:9031-9036
- ↑ Piotrowski et al. 2004. Physiology of Batrachochytrium dendrobatidis, a Chytrid Pathogen of Amphibians. Mycologia. 96:9-15
- ↑ 5.0 5.1 Fisher, M.C., T.W.J. Garner. 2020. Chytrid fungi and global amphibian declines. Nature Reviews Microbiology. (1740-1526).
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Rosenblum et al. 2010. The Deadly Chytrid Fungus: A Story of an Emerging Pathogen. PLoS Pathogens. 6(1):e1000550
- ↑ Bradley et al. 2019. Shifts in temperature influence how Batrachochytrium dendrobatidis infects amphibian larvae. Health and Medicine. 14:(9)
- ↑ 8.0 8.1 Stevenson et al. 2013. Variation in Thermal Performance of a Widespread Pathogen, the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis. PLoS ONE. 8(9):e73830
- ↑ Rooij et al. 2015. Amphibian chytridiomycosis: A review with focus on fungus-host interactions. Veterinary Research. 46(1):137.
- ↑ 10.0 10.1 Longcore et al. 1999. Batrachochytrium Dendrobatidis gen. et sp. nov., a Chytrid Pathogenic to Amphibians. Mycologia. 91:219-227
- ↑ [https://books.google.com/books?id=9IWaqAOGyt4C&pg=PA221&lpg=PA221&dq=Rhizophydiales+or+Chytridiales&source=bl&ots=1FA01hOf7B&sig=ACfU3U35iRTDCj6K4rWojQRwoh3Z77wn7A&hl=en&sa=X&ved=2ahUKEwiCjOC0r43pAhUDrJ4KHT8_DQsQ6AEwEXoECAkQAQ#v=onepage&q=Rhizophydiales%20or%20Chytridiales&f=false/ Margulis and Chapman. 2009. Chytridiomycota. In: Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Academic Press, Cambridge, pp.220-221.
- ↑ 12.0 12.1 12.2 12.3 Greenspan et al. 2012. Host invasion by Batrachochytrium dendrobatidis: fungal and epidermal ultrastructure in model anurans. Dis Aquat Org. 100:201-210
- ↑ Muletz et al. 2014. Unexpected Rarity of the Pathogen Batrachochytrium dendrobatidis in Appalachian Plethodon Salamanders: 1957–2011. PLOS. 9:e103728
- ↑ 14.0 14.1 Nichols et al. 2001. EXPERIMENTAL TRANSMISSION OF CUTANEOUS CHYTRIDIOMYCOSIS IN DENDROBATID FROGS. BioOne. 37(1):1-11
- ↑ [https://www.ncbi.nlm.nih.gov/pubmed/21816807/ Rollins-Smith et al. 2011. Amphibian Immune Defenses against Chytridiomycosis: Impacts of Changing Environments. Integrative and Comparative Biology. 51(4):552-562}
- ↑ Bower et al. 2017. Amphibians on the brink: Preemptive policies can protect amphibians from devastating fungal diseases. Science. 357:454-455
- ↑ O’Hanlon et al. 2018. Recent Asian origin of chytrid fungi causing global amphibian declines. Science. 360:621-627
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
